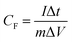DOI:
10.1039/C6RA10219K
(Paper)
RSC Adv., 2016,
6, 72845-72851
Application of novel multiple-dimensional cobalt oxides as the electroactive material on supercapacitors†
Received
20th April 2016
, Accepted 17th July 2016
First published on 18th July 2016
Abstract
Cobalt oxide is one of the attractive materials for supercapacitors (SCs) applying a faradaic reaction to store electrons. Researchers are devoted to synthesizing novel structures of cobalt oxides with different dimensions and designing layer-by-layer configurations composed of different morphologies to pursue multiple functions for attaining a better performance of the SC electrode. In this study, a two-step hydrothermal reaction is used to synthesize a cobalt oxide nanostructure composed of one, two, and three dimensional nanostructures on nickel foam. Superior to the traditional layer-by-layer configuration, this method is advantageous because it simultaneously synthesizes nanomaterials with multiple dimensions on the substrate, instead of combining the nanomaterials with different dimensions after each material has been synthesized separately. A one-dimensional (1D) cobalt oxide nanostem underlayer possesses speedy straight pathways for charge transfer, and three-dimensional (3D) nanoflowers composed of two-dimensional (2D) nanopetals distributed uniformly on top of the nanostem array provide a large active surface area for faradaic reactions. A high specific capacitance (CF) value of 916.9 F g−1 is obtained for the corresponding SC electrode using the cyclic voltammetry (CV) technique at a scan rate of 20 mV s−1. The results provide a new concept to in situ combine nanomaterials with multiple dimensions at once, avoiding time-consuming issues and adherence problems at the interface between different structures.
1. Introduction
Due to serious generation of pollution and considerable energy consumption, it is very important to develop environmentally friendly devices for energy generation and storage to solve these issues. Supercapacitors (SCs) have been regarded as one of the most important energy-storage devices, owing to their high energy and power densities as well as their long cycling life.1,2 Among SCs, the pseudocapacitor can provide even larger capacitances by utilizing fast redox kinetics, as compared with those generated by using electrochemical double layered capacitors (EDLCs), another type of SC. Cobalt-based oxides, sulfides, or hydroxides have attracted much attention as electroactive materials for the pseudocapacitor. The theoretical capacitance for cobalt-based compounds, i.e., oxides, sulfides and hydroxides, is estimated to be higher than 3000 F g−1. Also, cobalt-based compounds have been easily fabricated over the past decades.3–12 The specific capacitances reported in the literature are quite different. The main reason for this is the differences in the morphology of the nanomaterials. As the active material for a SC electrode, higher surface area and high conductivity are indispensable for accumulating and transferring more charges, respectively. The literature also proposed the same concept that the capacitive phenomenon is directly associated with surface properties, and any change in the surface morphology of the sample greatly influences its electrochemical performance.13 Hence, the synthesizing method is not directly related to the specific capacitance of a SC electrode, but the surface properties of the active material have a great impact on the specific capacitance of a SC electrode. In recent years, numerous studies have been devoted to constructing nanostructures with desirable morphologies and sizes to obtain high performances of SCs. One-dimensional (1D),14,15 two-dimensional (2D),6,16 and even three-dimensional (3D) nanostructures7,17 have been proposed with promising features as attractive charge-accumulating materials for providing high capacitances for the corresponding SCs. Zhang et al. used surfactants to synthesize different 1D Co3O4 nanowires on Ni foam via a hydrothermal process. The SC electrode with paddy-like Co3O4 nanowires showed the highest specific capacitance (CF) value of 1217.4 F g−1 measured by using a galvanic charge/discharge (GC/D) plot at a current density of 0.7 A g−1.14 Xia et al. reported a facile hydrothermal method for the large-area growth of 1D self-supported Co3O4 nanowire arrays. A CF value of 599.0 F g−1 at a current density of 2 A g−1 and an excellent cycle life were obtained for the corresponding SC electrode.15 The good performance of the SC electrode with 1D cobalt oxide nanomaterials as the electroactive material is mainly due to the high electrical conductivity of the 1D electron transfer path. Wang et al. synthesized Co3O4 ultrathin nanosheets with a high surface area of 97.2 m2 g−1 to obtain a CF value of 378.0 F g−1 at a current density of 1 A g−1.16 Kazemi et al. introduced an electrochemical fabrication of Co3O4 nanoflakes on an electro-etched carbon fiber by using a cathodic potential step method and obtained a CF value of 598.9 F g−1 at a current density of 6.25 A g−1 as well as high energy (40.75 W h kg−1) and power densities (27.69 kW kg−1).6 These literatures reported the synthesis of 2D cobalt oxide nanomaterials as the electroactive material for SCs with high surface areas as the electroactive sites for conducting more faradaic reactions. Also, 3D cobalt oxide nanostructures were proposed to obtain special features, for example, the suitable size of the pores for ion diffusion or the small protuberances on the surface of the 3D nanostructure for increasing the active sites. Liu et al. prepared porous Co3O4 nanospheres composed of nanosheets as the electroactive material for SCs and achieved a CF value of 246.7 F g−1 at a current density of 0.5 A g−1 owing to the desirable morphology.17 Zhou et al. synthesized hollow fluffy Co3O4 cages as the electroactive material for applications in SCs and the oxygen evolution reaction. A CF value of 948.9 F g−1 was achieved at a current density of 1 A g−1 for the pertinent SC electrode, due to the efficient interaction between the electrolyte and the electroactive component as well as the many active sites for the surface redox reactions.7
Nevertheless, the literature relating to the simultaneous synthesis of 1D, 2D, and 3D nanocomposites on the substrate and their electrocapacitive performance for the pertinent SC electrode is limited. Lin et al. combined 1D and 3D ZnO nanostructures by using a layer-by-layer method to deposit nanoparticles on a nanorod array and obtain a large surface area and straight electron transfer path. However, the 1D and 3D nanostructures were synthesized separately and combined in extra steps.18 The same group also proposed the synthesis of a composite composed of 1D and 3D TiO2 nanostructures on a Ti foil substrate. The 1D TiO2 nanotube array (TiNA) was made by applying the anodization method and the 3D TiO2 nanoparticles were deposited inside and on top of the TiNA by using a dip-coating method under vacuum conditions.19 The nanocomposite composed of the 1D and 3D nanostructures was also made by synthesizing the 1D and 3D nanostructures separately and combining them via a post treatment. In all, nanostructures composed of multiple dimensions were usually synthesized by making the different nanostructures separately and then combining all of the materials in the final stage. To the best of our knowledge, this is the first study reporting a method to synthesize cobalt oxide nanostructures with multiple dimensions arranged in a regular order at the same time by using a two-step hydrothermal reaction, instead of synthesizing 1D, 2D, and 3D nanostructures separately and combining all of the nanomaterials in an extra final step. This idea is very promising since the adherence and uniformity of the nanostructures with different dimensions can be largely improved as compared with those synthesized using the traditional method, i.e., making the nanomaterials separately and combining all of the structures in an extra step like the layer-by-layer configuration. The cobalt oxide nanostructure was synthesized via a two-step hydrothermal method, in which 1D, 2D, and 3D nanostructures were successfully made on Ni foam at the same time. The 1D nanostructure was grown directly on the Ni foam as the underlayer, and clusters of 3D nanoflowers composed of 2D nanopetals with numerous nanowrinkles on the surface were obtained as the overlayer. This novel nanostructure is expected to have all of the advantages of 1D, 2D, and 3D nanostructures. A large CF value of 916.9 F g−1 was obtained for the pertinent SC electrode, evaluated by cyclic voltammetry (CV) measurements at a scan rate of 20 mV s−1, due to the vertical growth of the 1D nanostem in the underlayer providing vertical charge transferring paths as well as the large active areas of the 2D nanopetal-composed 3D nanoflowers and the nanowrinkles on the nanopetals conducting more faradaic reactions. The high-rate charge/discharge capability was also obtained with CF values of 1155.6 and 888.9 F g−1 by using the galvanic charge/discharge measurement respectively at current densities of 1 and 8 A g−1. There was only a 21.5% decay when an 8-fold current density was applied for the measurement. The hydrothermal reaction is the most commonly used method to synthesize nanomaterials due to its simple and low-cost properties,20–22 and the reaction temperature, reaction duration, as well as the precursor concentrations can be easily tuned for synthesizing well-defined nanostructures to achieve high performing active materials. In addition, the active material can grow on the surface of the substrate directly during the synthesizing process of the hydrothermal reaction. This is beneficial for contact between the active material and the substrate. The method for synthesizing the nanomaterial was chosen in consideration of the cost of the instrument and the chemicals required for applying the method, as well as the time and safety for conducting the whole synthesizing process. Dam et al. synthesized cobalt oxide microdumbbells by using a multistep hydrothermal method with a hexanitrocobaltate complex as a cobalt source, and a surface area of 70.8 m2 g−1 and CF value of 407.5 F g−1 were obtained for the pertinent SC electrode.23 The precursor for the cobalt ion is different from that used in our work, so the morphology is quite varied as compared with the sample synthesized in our experiment. A microwave method has also been applied for synthesizing cobalt oxide nanostructures. Vijayakumar et al. synthesized cobalt oxide nanoparticles through a microwave method for SC studies. A CF value of 519 F g−1 was obtained from charge–discharge studies.24 However, the cobalt oxide nanostructures are not synthesized on the substrate directly in the two studies mentioned above. Therefore, the specific capacitances are lower than that obtained in our work. In addition, Kung et al. prepared Co3O4 nanosheets by using an electrodeposition method and UV-ozone treatment. A CF value of 1033.3 F g−1 was therefore obtained for the pertinent SC electrode at a current density of 2.5 A g−1, due to the large surface area of the nanosheet.25 This method is more complicated than the hydrothermal method used in our work, and the requirement for the instrument is also higher than that of the hydrothermal reaction. In addition, some of the studies used surfactants to synthesize novel structures with large surface area and high conductivity.26 However, in our work, there is no need to add a surfactant for designing the well-defined nanostructure.
2. Experimental section
2.1. Materials
Ammonium fluoride (NH4F, ≥98.0%), ethanol (EtOH, ≥99.8%), hydrochloric acid (HCl, analytical grade), and urea (CO(NH2)2, BioReagent) were obtained from Sigma-Aldrich. Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O, 98.0%) and hexamethylenetetramine (C6H12N4, HMT, 100%) were purchased from Showa.
2.2. Preparation of supercapacitor electrodes with a cobalt oxide nanostructure on Ni foam
The nickel foam substrate was cut to the size of 1 cm × 3 cm, and then cleaned by soaking in 6 M HCl solution for 30 min under ultrasonic vibration to remove the oxides on the surface. Subsequently the nickel foam was alternatively washed with deionized water (DIW) and EtOH, and then the cleaned nickel foam was dried in a vacuum oven for 3 h. The cobalt oxide nanostructures were synthesized using a two-step hydrothermal method. In the first hydrothermal step, 0.58 g of Co(NO3)2·6H2O, 0.30 g of NH4F, and 0.60 g of CO(NH2)2 were dissolved in 36 ml of DIW under stirring. Then 10 ml of the above solution was dropped into a 110 ml Teflon-lined stainless steel autoclave along with the clean nickel foam leaned against the wall of the liner. The autoclave was then heated to 100 °C and kept at the same temperature for 4 h, and subsequently cooled naturally to room temperature. The as-prepared sample was washed with DIW and then annealed at 250 °C for 3 h at a heating and cooling rate of 5 °C min−1. The second hydrothermal step was then applied as follows. 0.58 g of Co(NO3)2·6H2O and 0.56 g of HMT were dissolved in 36 ml of DIW under stirring. Then 10 ml of the above solution was dropped into a 110 ml Teflon-lined stainless steel autoclave along with the first hydrothermal reaction treated nickel foam leaned against the wall of the liner. The autoclave was then heated to 100 °C and kept at the same temperature for 12 h, and subsequently cooled naturally to room temperature. The as-prepared sample was washed with DIW and EtOH alternatively and then annealed at 250 °C for 3 h at a heating and cooling rate of 5 °C min−1. The loading mass of the active material on the electrode is estimated to be 3 mg. The loading mass was measured by a METTLER TOLEDO® balance with an accuracy of 0.01 mg. The mass of the electroactive material is the mass difference of the SC electrode before and after the deposition of the electroactive material, i.e., the mass of the bare nickel foam subtracted from the total mass of the SC electrode.
The mechanism for the growth of cobalt oxide was proposed as follows. During the first hydrothermal reaction, Co(NO3)2, NH4F, and CO(NH2)2 were dissolved in DIW to release Co2+, F−, and OH− ions as is shown in eqn (1)–(3). Then the Co2+, F−, and OH− ions formed the precipitation of Co(OH)F as is presented in eqn (4) and (5). The post-annealing process made Co(OH)F decompose to release HF gas and produce the CoO nanostructure, as is shown in eqn (6). The nanostructure was formed during the second hydrothermal reaction. It was reported that thinner nanosheet arrays can be obtained by applying a secondary hydrothermal reaction to treat the samples with additional Co2+ salts and appropriate bases (e.g. urea or HMT) through a process involving the dissolution and recrystallization of hexagonal nanosheet arrays.27 Hence, HMT and Co(NO3)2 probably act as the structure-directing agent and ingredient for the growth of the cobalt compound, respectively.
| | |
Co(NO3)2 → Co2+ + 2NO3−
| (1) |
| | |
CO(NH2)2 + 3H2O → 2NH4+ + CO2 + 2OH−
| (3) |
2.3. Material characterization and electrochemical measurements
The morphology of the cobalt oxide was observed by field-emission scanning electron microscopy (FE-SEM, Nova NanoSEM 230, FEI, Oregon, USA), transmission electron microscopy (TEM, JEM-1230, JEOL, Tokyo, Japan), and high-resolution transmission electron microscopy (HRTEM, Philips Tecnai F30 Field Emission Gun Transmission Micro-scope (FEG-TEM)). The composition of the samples was investigated by using X-ray diffraction (XRD, X’Pert3 Powder, PANalytical) patterns. The CV and GC/D plots were measured by using a potentiostat/galvanostat (PGSTAT 204, Autolab, Eco-Chemie, the Netherlands) carried out with a three-electrode electrochemical system, where the cobalt oxide nanostructure electrode was used as the working electrode, a Pt wire was used as the counter electrode, and an Ag/AgCl/saturated KCl electrode was used as the reference electrode in 2 M KOH solution.
3. Results and discussion
3.1. Morphology and composition of the cobalt oxide nanostructure
The morphology of the cobalt oxide nanostructure was first examined by using SEM images, as is shown in Fig. 1. The lowest magnification of the SEM image presents the fully covered nanomaterials on the Ni foam with different morphologies in the two layers, as is show in Fig. 1(a). Fig. 1(b) shows the magnified image of Fig. 1(a). Two different morphologies were obviously observed. The 1D nanostems were uniformly grown in the underlayer, and several 3D nanoflowers were observed to be deposited on top of the nanostem array as the overlayer. A closer observation was made in Fig. 1(c). The nanoflower is composed of 2D nanopetals with a width larger than 1 μm in the surrounding area and a width smaller than 100 nm at the center. The magnified image of the nanopetals is shown in Fig. 1(d). The surface of the nanopetals is very rough with several wrinkles randomly distributed. The cross-sectional SEM image was also shown in Fig. S1(a) in the ESI.† To illustrate the 1D nanostem underlayer and the 3D nanoflower overlayer more clearly, Fig. S1(b) presents the same SEM image as Fig. S1(a)† but with cartoons of the 3D nanoflowers and 1D nanostems inserted. The 3D nanoflower overlayer was not fully distributed on the 1D nanostem underlayer. Several nanostems were uncovered and directly exposed to the electrolyte, while all of the 3D nanoflowers were found to stand on the underlayer of the 1D nanostems. That is, for the region with a presence of 3D nanoflowers, the underlayer of the 1D nanostem and the overlayer of the 3D nanoflowers can be obviously observed, but for other regions without the growth of the 3D nanoflowers, there is only one layer of nanostems that can be observed. The second hydrothermal reaction is necessary for synthesizing the multi-dimensional nanostructure. The cobalt oxide nanostructure synthesized before applying the second hydrothermal reaction was examined in the SEM image shown in Fig. S2 in the ESI.† It was clearly observed that only a nanopillar structure was obtained for cobalt oxide if a one-step hydrothermal reaction was applied. In addition, to further observe the inner morphology of the cobalt oxide nanostructure, the TEM images of the 1D nanostem and the 2D nanopetal are shown in Fig. 2(a) and (c), respectively, and the corresponding HRTEM images are presented in Fig. 2(b) and (d). The 1D nanostem is densely packed with tiny nanoparticles with a diameter of around 6 to 10 nm, as is shown in Fig. 2(a) and (b). To more clearly illustrate the estimation of the particle sizes, the TEM image along with inserted lines for estimating the sizes is shown in Fig. S3 in the ESI.† As for the 2D nanopetals, a perfect 2D structure was successfully synthesized instead of being composed of smaller nanomaterials. Several cracks were found to appear on the surface of the 2D nanopetals with a size of 1 to 2 nm in width and 5 to 15 nm in length. The pores and cracks respectively in the 1D nanostem and on the 2D nanopetal are probably suitable for ion diffusion to enhance the faradaic reactions and therefore to improve the electrocapacitive performance of the SC electrode.
 |
| | Fig. 1 The SEM image of the cobalt oxide nanostructure grown on Ni foam. | |
 |
| | Fig. 2 (a) The TEM and (b) HRTEM images of the cobalt oxide nanostem and (c) the TEM and (d) HRTEM images of the cobalt oxide nanopetal in the cobalt oxide nanostructure. | |
The composition of the cobalt oxide is investigated by using the XRD pattern as is shown in Fig. 3(a). The two strongest peaks at around 45° and 53° are attributed to the current collector of the Ni foam, while the characteristic diffraction peaks at the plane of (111), (200), (220), (311), and (222) could be indexed to CoO,28 indicating the successful synthesis of CoO via this two-step hydrothermal reaction. Also, the energy dispersive X-ray (EDX) spectrum was measured for the sample without the current collector, Ni foam, as is shown in Fig. 3(b). The EDX spectrum was obtained directly using the cobalt oxide/nickel foam electrode. To reduce the signal from the Ni foam substrate, the signals for the EDX spectrum were collected in a very short period. The signals from the nickel foam substrate are negligible. Therefore, the EDX spectrum only presents Co and O signals from the active material, the cobalt oxide. The spectrum again confirms the composition of pure cobalt and oxygen for this nanostructure.
 |
| | Fig. 3 (a) The XRD pattern and (b) EDX spectrum for the cobalt oxide nanostructure. | |
3.2. The electrochemical performance of the supercapacitor electrode with cobalt oxide nanostructure
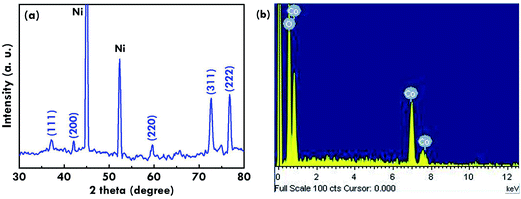
To evaluate the performance of the SC electrode with the cobalt oxide nanostructure as the electroactive material, the corresponding CF value was estimated by using a CV plot in the range of −0.2 to 0.6 V, as is shown in Fig. 4(a). The CF value was calculated based on eqn (7) as follows.29| |
 | (7) |
where  is the integrated area in the cathodic part of the CV plot, I is the current, ν is the scan rate, ΔV is the potential window, and m is the mass of the electroactive material. The pseudo-capacitive behaviors were obviously observed with one couple of the redox peaks for the faradaic reactions presented in eqn (8) as follows.
is the integrated area in the cathodic part of the CV plot, I is the current, ν is the scan rate, ΔV is the potential window, and m is the mass of the electroactive material. The pseudo-capacitive behaviors were obviously observed with one couple of the redox peaks for the faradaic reactions presented in eqn (8) as follows.| |
 | (8) |
 |
| | Fig. 4 (a) The CV curves measured at different scan rates, (b) the CF value calculated from the CV curves as a function of the corresponding scan rate, (c) the GC/D plots measured at different current densities, and (d) the CF value calculated from the GC/D plots as a function of the corresponding current for the SC electrode with the cobalt oxide nanostructure as the electroactive material. | |
A high CF value of 916.9 F g−1 was obtained at a scan rate of 20 mV s−1, suggesting the outstanding performance of the cobalt oxide nanostructure as the electroactive material for the SC electrode. The peak separation became larger and larger when the scan rate applied on the measurement was increased. This is a common phenomenon in the electrochemical field. Also, CF values of 643.0, 358.4, and 180.5 F g−1 were respectively obtained for the cases measured at scan rates of 40, 80, and 160 mV s−1. To evaluate the high-rate charge/discharge capacity, the CF value measured by using the CV plots as a function of scan rate is further shown in Fig. 4(b). It is found that when the scan rate increased by 8-fold, the CF value still remains as 28% of the initial value, suggesting that the performance of this SC electrode may not be seriously reduced even if a high rate charge/discharge process was applied. In addition, the GC/D plots for the SC electrodes with the cobalt oxide nanostructure were measured at different current densities in a charging/discharging voltage range of 0 to 0.45 V, as is presented in Fig. 4(c). The values of CF were calculated based on eqn (9) as follows.29
| |
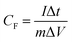 | (9) |
where
I is the current, Δ
t is the discharge time,
m is the weight of the active material in the electrode, and Δ
V is the potential window during the charge/discharge process. The highest
CF value of 1155.6 F g
−1 was achieved at a current of 1 A g
−1. The charge and discharge curves were found to be very symmetric for all of the cases, even when the current density was applied as high as 8 A g
−1, suggesting good reversibility for this CoO-based SC electrode.
CF values of 1133.3, 977.8, and 888.9 F g
−1 were obtained at current densities of 2, 4, and 8 A g
−1, respectively. The
CF value as a function of the current density is shown in
Fig. 4(d) to evaluate the high-rate operating ability. The decrease of the
CF value measured at higher currents is limited as is seen in this plot. When the current density was enhanced by 8-fold from 1 to 8 A g
−1, a 78.4% retention of the
CF value was still obtained, again suggesting the permanence of this SC electrode at a high-rate operating process. The high
CF value for this CoO-based SC electrode can be attributed to multiple functions from the 1D, 2D and 3D morphology-combined nanostructure. The scheme for presenting the nanostructure with the 1D nanostem as the underlayer and the 3D nanoflower composed of the 2D nanopetals as the overlayer is shown in
Fig. 5 to more clearly demonstrate the functions of each layer. The 1D nanostem has a straight charge transfer pathway to accelerate the electron transport from the overlayer generated by the faradaic reactions to the current collector, the nickel foam. The 3D nanoflowers can provide large amounts of active sites for conducting faradaic reactions since they are composed of 2D nanopetals which have a large surface area over the 2D structure, and the numerous wrinkles on the surface of the 2D nanopetals can even further enhance the surface area. The overlayer is made by using the material with a large surface area and the underlayer is synthesized by applying the material with a speedy charge transfer path. Hence, this nanostructure is very promising as an electroactive material for the SC electrode. The SEM images of the 3D nanoflowers with different growth extents are also shown in this scheme. It was observed that the growth extent of the nanoflower varied depending on the location of the nickel foam. This phenomenon may be due to the different concentrations of the precursor solution on the different regions of the Ni foam, assuming the concentration of the ions is not totally the same throughout. The nanoflower seems to bloom gradually from the left to the right SEM images, suggesting that large nanopetals may form in the earlier stage and subsequently be consumed to become smaller nanopetals during the process.
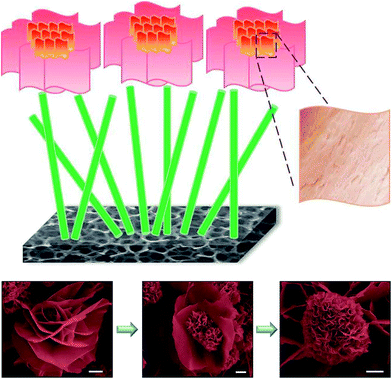 |
| | Fig. 5 The scheme for presenting the CoO nanostructure with the 1D nanostem at the underlayer and the 3D nanoflower composed of 2D nanopetals at the overlayer along with SEM images of the nanoflowers with a scale bar of 1 μm. | |
Last but not least, the cycling stability of the SC electrode with the cobalt oxide nanostructure as the electrocapacitive material was evaluated by measuring the repeated GC/D curves 2000 times. Fig. 6(a) shows the first and last four charge/discharge curves of the 2000 times repeated charge/discharge process. The symmetric charge and discharge curves were observed in the first and last four curves, suggesting higher reversibility for this case. The CF value still had a 75% retention at the 2000th cycle, as compared with the value obtained at the first cycle. The cycling stability is expected to be further improved by incorporating carbon materials in the electrode. This will be done in our future studies. Also, the CF value retention as compared with that obtained during the first charge/discharge cycle as well as the coulombic efficiency as a function of the cycling number was calculated and is presented in Fig. 6(b). The CF value retention dropped to a large extent during the first 200 cycles, and remained nearly constant for the last 800 cycles, which kept the retention higher than 75% during the whole process. Also, a coulombic efficiency higher than 90% was achieved during the entire measurement.
 |
| | Fig. 6 (a) The GC/D plots of the first and last four charge/discharge curves in the 2000 times repeated charge/discharge process, and (b) the CF value and the coulombic efficiency as a function of the cycling number for the SC electrode with the cobalt oxide nanostructure as the electroactive material. | |
4. Conclusions
A cobalt oxide nanostructure composed of 1D nanostems and 2D nanopetal-assembled 3D nanoflowers was simultaneously synthesized on nickel foam by using a facile two-step hydrothermal reaction. The 1D nanostems were packed with tiny nanoparticles with a diameter of several nanometers. The 2D nanopetals presented perfect 2D structures with numerous wrinkles on the surface. A high CF value of 916.9 F g−1 was obtained by using a CV plot with a scan rate of 20 mV s−1. A high-rate charge/discharge capability was achieved with a 78.4% retention of the CF value when the current density for measuring the GC/D was increased from 1 to 8 A g−1. The outstanding performance of the SC electrode with the cobalt oxide nanostructure as the electroactive material is due to the 1D charge transfer pathway of the underlayer for speedy electron transportation and the large electroactive surface area of the 2D and 3D overlayer for conducting more faradaic reactions.
Acknowledgements
This work was supported in part by the Ministry of Science and Technology of Taiwan, under the grant numbers: MOST 103-2218-E-027-010-MY2 and MOST 103-2119-M-027-001-.
References
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- R. Kötz and M. Carlen, Electrochim. Acta, 2000, 45, 2483–2498 CrossRef.
- S. Bae, J. H. Cha, J. H. Lee and D. Y. Jung, Dalton Trans., 2015, 44, 16119–16126 RSC.
- K. Qiu, H. Yan, D. Zhang, Y. Lu, J. Cheng, W. Zhao, C. Wang, Y. Zhang, X. Liu, C. Cheng and Y. Luo, Electrochim. Acta, 2014, 141, 248–254 CrossRef CAS.
- F. Luo, J. Li, Y. Lei, W. Yang, H. Yuan and D. Xiao, Electrochim. Acta, 2014, 135, 495–502 CrossRef CAS.
- S. H. Kazemi, A. Asghari and M. A. kiani, Electrochim. Acta, 2014, 138, 9–14 CrossRef CAS.
- X. Zhou, X. Shen, Z. Xia, Z. Zhang, J. Li, Y. Ma and Y. Qu, ACS Appl. Mater. Interfaces, 2015, 7, 20322–20331 CAS.
- S. Abouali, M. Akbari Garakani, B. Zhang, Z. L. Xu, E. Kamali Heidari, J. Q. Huang, J. Huang and J. K. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 13503–13511 CAS.
- Y. Z. Zhang, Y. Wang, Y. L. Xie, T. Cheng, W. Y. Lai, H. Pang and W. Huang, Nanoscale, 2014, 6, 14354–14359 RSC.
- J. M. Chiu, L. Y. Lin, P. H. Yeh, C. Y. Lai, K. Teng, C. C. Tu, S. S. Yang and J. F. Yu, RSC Adv., 2015, 5, 83383–83390 RSC.
- F. Luo, J. Li, H. Yuan and D. Xiao, Electrochim. Acta, 2014, 123, 183–189 CrossRef CAS.
- K. J. Huang, J. Z. Zhang, G. W. Shi and Y. M. Liu, Mater. Lett., 2014, 131, 45–48 CrossRef CAS.
- Y. Hao, H. Wang, Z. Hu, L. Gan and Z. Xu, New J. Chem., 2015, 39, 68–71 RSC.
- X. Zhang, Y. Zhao and C. Xu, Nanoscale, 2014, 6, 3638–3646 RSC.
- X. H. Xia, J. P. Tu, Y. J. Mai, X. L. Wang, C. D. Gu and X. B. Zhao, J. Mater. Chem., 2011, 21, 9319 RSC.
- X. Wang, S. Yao, X. Wu, Z. Shi, H. Sun and R. Que, RSC Adv., 2015, 5, 17938–17944 RSC.
- R. Liu, Z. Jiang, Q. Liu, X. Zhu and W. Chen, CrystEngComm, 2015, 17, 4449–4454 RSC.
- L. Y. Lin, M. H. Yeh, C. P. Lee, C. Y. Chou, R. Vittal and K. C. Ho, Electrochim. Acta, 2012, 62, 341–347 CrossRef CAS.
- L. Y. Lin, M. H. Yeh, C. P. Lee, Y. H. Chen, R. Vittal and K. C. Ho, Electrochim. Acta, 2011, 57, 270–276 CrossRef CAS.
- L. Lin, J. Liu, T. Liu, J. Hao, K. Ji, R. Sun, W. Zeng and Z. Wang, J. Mater. Chem. A, 2015, 3, 17652–17658 CAS.
- W. Kong, C. Lu, W. Zhang, J. Pu and Z. Wang, J. Mater. Chem. A, 2015, 3, 12452–12460 CAS.
- Y. Zhang, M. Ma, J. Yang, C. Sun, H. Su, W. Huang and X. Dong, Nanoscale, 2014, 6, 9824–9830 RSC.
- D. T. Dam and J. M. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 20729–20737 CAS.
- S. Vijayakumar, A. K. Ponnalagi, S. Nagamuthu and G. Muralidharan, Electrochim. Acta, 2013, 106, 500–505 CrossRef CAS.
- C. W. Kung, H. W. Chen, C. Y. Lin, R. Vittal and K. C. Ho, J. Power Sources, 2012, 214, 91–99 CrossRef CAS.
- F. Manteghi, S. H. Kazemi, M. Peyvandipour and A. Asghari, RSC Adv., 2015, 5, 76458–76463 RSC.
- Q. Yang, Z. Lu, X. Sun and J. Liu, Sci. Rep., 2013, 3, 3537 Search PubMed.
- C. Shang, S. Dong, P. Hu, J. Guan, D. Xiao, X. Chen, L. Zhang, L. Gu, G. Cui and L. Chen, Sci. Rep., 2015, 5, 8335 CrossRef CAS PubMed.
- L. Y. Lin, M. H. Yeh, J. T. Tsai, Y. H. Huang, C. L. Sun and K. C. Ho, J. Mater. Chem. A, 2013, 1, 11237 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10219k |
| ‡ These authors contributed equally. |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 

 is the integrated area in the cathodic part of the CV plot, I is the current, ν is the scan rate, ΔV is the potential window, and m is the mass of the electroactive material. The pseudo-capacitive behaviors were obviously observed with one couple of the redox peaks for the faradaic reactions presented in eqn (8) as follows.
is the integrated area in the cathodic part of the CV plot, I is the current, ν is the scan rate, ΔV is the potential window, and m is the mass of the electroactive material. The pseudo-capacitive behaviors were obviously observed with one couple of the redox peaks for the faradaic reactions presented in eqn (8) as follows.