Largely enhanced electrical properties of polymer composites via the combined effect of volume exclusion and synergy
Kai Wua,
Linyu Wua,
Weixing Yanga,
Songgang Chaib,
Feng Chen*a and
Qiang Fu*a
aCollege of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, China. E-mail: fengchen@scu.edu.cn; qiangfu@scu.edu.cn; Fax: +86-28-85461795; Tel: +86-28-85460690 Tel: +86-28-85461795
bGuangdong Shengyi Technology Limited Corporation, Dongguan, 523039, China
First published on 23rd May 2016
Abstract
It has been demonstrated that introducing another non-conductive particle into conductive filler filled composites could result in a better conductivity due to a volume exclusion effect. It was also reported that combining electrically conductive fillers with different geometric shapes and aspect ratio together could enhance the electrical conductivity due to the synergistic effect. To further explore these two effects on the electrical property enhancement, we firstly encapsulated neat silica (SiO2) with graphene oxide (GO) through electrostatic self-assembly to render SiO2 with surface conductivity. Then neat SiO2 or SiO2@GO together with multi-walled carbon nanotubes (MWCNT) were mixed with polystyrene (PS) to prepare conductive composites. In this way, the volume exclusion effect in the PS/MWCNT/SiO2 system and combined effect of volume exclusion and synergy in the PS/MWCNT/SiO2@rGO system could be investigated and compared. An obvious increased conductivity was observed only in the transition composition region for the PS/MWCNT/SiO2 system compared with the PS/MWCNT system. However, a largely enhanced conductivity was achieved through the composition region for the PS/MWCNT/SiO2@rGO system compared with the PS/MWCNT/SiO2 system and PS/MWCNT, accompanied by great enhancement of the electromagnetic interference (EMI) shielding effectiveness. Given the SEM characterization and rheological properties, we attributed this obvious enhanced electrical property to both a volume exclusion effect and a synergistic effect.
1. Introduction
Conductive polymer composites (CPCs) have attracted comprehensive interest owing to their various applications, such as in strain and damage sensing, stretchable conductors, thermoelectric materials and electromagnetic shielding.1–13 CPCs are usually fabricated through incorporating single or hybrid conductive fillers (e.g. carbonaceous, conductive polymeric particles and metallic) with a polymer matrix. On increasing the filler concentration, the CPCs will exhibit an insulator/conductor transition, with a jump in electrical conductivity by several orders of magnitude. This phenomenon is defined as electrical percolation, and the critical fraction is termed the percolation threshold. A lot of work has been done to reduce the percolation threshold. For instance, designing polymer blends to selectively locate conductive fillers in one of the polymer phases or at the interfaces;14,15 using thermal annealing above the glass transition temperature or melting temperature of the polymer to induce the formation of a conductive network;16,17 using shear force to trigger orientation of conductive fillers;18,19 using polymer latex particles to form a segregated network;20,21 using an electric or magnetic field to control the morphology of the conductive network;22,23 preforming a highly porous and compact 3-D conductive framework in a polymer matrix.10Among these methods, volume exclusion effect via introducing another non-conductive particles, such as clay, silica and calcium carbonate, to form a segregated conductive structure is considered to be significant to decrease the percolation threshold.24–26 This is ascribed to the fact that the conductive fillers tend to be excluded from non-conductive particles dominated areas, leading to higher effective concentration of conductive fillers.25 However, the idea of volume exclusion does not always work, it depends on the size and volume content of non-conductive particles, the interaction between conductive and non-conductive particles, as well as the combination of conductive and non-conductive particles. For example, at the regions below or above percolation threshold, addition of non-conductive particles only results in a limited increase of conductivity, while a largely enhanced conductivity is observed at the vicinity of transition. On the other hand, it was also reported that mixing electrically conductive fillers with different geometric shapes and aspect ratio in polymer matrix may show synergistic effect that will increase the electrical conductivity of the composites.27–31 So far, the effect of volume exclusion and synergistic on the electrical conductivity of polymer composites has been investigated separately. If the non-conductive particles are coated with a thin layer of conductive material, one expects a combined effect of volume exclusion and synergy. How does the conductivity of CPCs change if adding surface coated non-conductive particles? Will it be extend to all the composition region for the enhancement of conductivity? These questions would be very interesting and worth to be explored.
For this reason, in this study, we firstly studied the volume exclusion effect of 20 wt% neat silica (SiO2) particles through incorporating them with multi-walled nanotubes (MWCNT) using PS as polymer matrix. Only at the percolation threshold, the electrical conductivity did exhibit increase with orders of magnitude. At other concentrations, a limited enhancement in electrical conductivity was obtained in comparison with that of PS/MWCNT, which was similar to the results in the previous study.25 Hence increasing the loading of electrically inert SiO2 was necessary for effectively enhance the electrical property according to previous report. In order to avoid introducing a mass of non-conductive particles in sacrifice of mechanical property and processability, core–shell structure of modified SiO2 was prepared which can render SiO2 with surface conductivity. To do this, the surface of SiO2 was wrapped with graphene oxide (GO) thin layer through mutual electrostatic interactions in the aqueous solution. The surface coated SiO2 was added again in to PS/MWCNT composites to investigate the combined effect of volume exclusion and synergy, since the GO layer could be thermally reduced via compression molding at high temperature. The effect of thermal reduction on GO was confirmed by TGA, FTIR and Raman spectra. As consequences, a largely enhanced conductivity was achieved in all the composition region for PS/MWCNT/SiO2@rGO system compared with PS/MWCNT/SiO2 system and PS/MWCNT, companied with greatly enhancement of electromagnetic interference (EMI) shielding effectiveness. SEM characterization and rheological test were carried out to understand the mechanism of property enhancement.
2. Experimental
2.1 Materials
Graphite powders were purchased from Qingdao Black Dragon graphite Co., Ltd. Micron sized silica (average diameter: 5 μm) and 3-aminopropyltriethoxysilane (APS) were purchased from Aladdin Chemicals Co., Ltd. Potassium permanganate (KMnO4), sulfuric acid (H2SO4 98%), hydrogen peroxide (H2O2), hydrochloric acid, ethanol and N,N-dimethylformamide (DMF) were supplied by Kelong Chemical reagent plant (Chengdu, China). Multi-walled carbon nanotubes (MWCNT, NC7000, 1.5 μm in length, diameter of 9.5 nm) were purchased from Nanocyl S.A., Belgium. Amorphous polystyrene (PS, PG33, 1.04 g cm−3) was purchased from Sino-foreign Joint Venture Zhenjiang Qimei Chemical Co., Ltd.2.2 Surface modification of SiO2 by APS and encapsulation by GO
Similar to previous studies,32 the core–shell structure of SiO2@GO hybrid was fabricated by two environmental steps: the surface modification of SiO2 particles with APS and the electrostatic self-assembly between GO and the modified SiO2.GO was prepared through the exfoliation of natural graphite by Hummers method.33 Then the exfoliated GO was collected by centrifugation to remove the nonexfoliated graphite. After that, steady GO aqueous solution was achieved by sonication in deionized water for 2 h.
The modification of SiO2 was prepared according to our previous study.34 In a typical process, according to Fig. 1(1), SiO2 particles (10 g) were dispersed well in 300 ml ethanol solution (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 95
H2O = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), being mixed with 0.5 ml of silane coupling agent APS. Then, the solution was kept at 60 °C overnight for reaction. After that, the obtained product SiO2–NH2 was washed by deionized water and ethanol for 2 times, and dried under vacuum.
5), being mixed with 0.5 ml of silane coupling agent APS. Then, the solution was kept at 60 °C overnight for reaction. After that, the obtained product SiO2–NH2 was washed by deionized water and ethanol for 2 times, and dried under vacuum.
The electrostatic self-assembly between GO and the SiO2–NH2 was achieved through a simple procedure, as is shown in Fig. 1(2). 1000 ml GO aqueous solution (0.15 mg ml−1) and 500 ml SiO2–NH2 suspension (20 mg ml−1) were mixed together under mild magnetic stirring for 2 h. Then, the SiO2@GO powder with the GO concentration of 1.5 wt% was collected by centrifugation and dried under vacuum.
2.3 Preparation of PS-based composites
For the fabrication of PS/MWCNT/SiO2@rGO, according to Fig. 1(3), the desired amount of fillers were dispersed well in DMF through mild magnetic stirring and sonication. Meanwhile, 10 g PS was dissolved in 50 ml DMF at 60 °C. Afterwards, the fillers suspension was mixed with PS solution well through intense mechanical stirring and then both fillers and PS were precipitated in deionized water. The collected precipitation was dried under vacuum and then processed into specimens by compression molding at 200 °C for 40 min, simultaneously thermally reducing GO to rGO. PS, PS/MWCNT, PS/MWCNT/SiO2 nanocomposites were prepared in a similar solution.2.4 Characterization
Scanning electron microscopy (SEM) images of silica were obtained by using the scanning electron microscopy (SEM, Inspect F, FEI Company, USA). The cryo-fractured surfaces of PS/MWCNT/SiO2 and PS/MWCNT/SiO2@rGO nanocomposites were characterized to observe the morphology. The fractured surfaces were prepared in liquid N2 and were sputtered with gold in vacuum prior to observation.Energy dispersive X-ray spectrometry (EDS) was utilized to analyze the composition of elements in GO, SiO2, SiO2–NH2, SiO2@GO to prove the encapsulation of GO on the surface of SiO2 particles.
Zeta potential measurements were characterized using a Zetasizer 3000 (Malvern Instruments). Before measurement, GO and silica aqueous solution were respectively diluted to 0.05, 2 mg ml−1.
Raman spectra of GO and SiO2@GO were characterized with a laser wavelength of 532 nm.
Fourier transform infrared (FTIR, Nicolet 6700) spectroscopy was carried out to characterize the functional groups in GO and rGO to discuss the influence of thermal reduction on GO.
The thermal stability and thermal reduction influence was studied with Thermal Gravimetric Analysis (TGA, TA instrument, Q500, USA). The temperature increased from 30 to 600 °C for composites and 30 to 760 °C for GO at a heating rate of 10 °C min−1 in nitrogen atmosphere.
The electrical conductivity of the samples was characterized with a Keithley 6487 picoammeter under a constant voltage of 1 V in order to avoid strong electric current within the specimen. To prevent contact resistance, silver paint was brushed on the both ends of the samples.
Melt rheology of PS, PS/MWCNT/SiO2 and PS/MWCNT/SiO2@rGO nanocomposites was measured in a strain-controlled dynamic rheometer Bohlin Gemini 200, Malvern Instruments, British. In order to achieve a linear viscoelastic response, the frequency sweep was set in the range of 0.01–100 Hz with the strain amplitude of 1%. Disk-shaped samples from compression molding with the diameter of 25 mm and thickness of 18 mm were used during this characterization.
EMI shielding characteristics of the samples with the diameter of 13 mm and thickness of 1.3 mm report here were measured by us using a coaxial test cell (APC-7 connector) connected with an Agilent N5230 vector network analyzer.
3. Results and discussion
3.1 Electrostatic assembly of GO and APS-modified SiO2
Two-dimensional GO nanosheets ionized negative charge in aqueous solution owing to large amount of carboxylic acid group on their surface. Driven by the mutual electrostatic interactions, the sheets can encapsulate many positively charged particles when mixed them in aqueous solution together.32,35,36 In this study, in order to make SiO2 particles carry groups which can ionized positive charge in aqueous solution, they were modified by APS. As a result, amino groups (-NH2) were grafted onto the surface of SiO2 particles. In aqueous solution with the pH value of 7.0, the surface charge of GO, SiO2 and SiO2–NH2 was respectively characterized by zeta potential tests. GO and unmodified SiO2 both had highly negative surface charge with ξ potential value of −57.80 mV and −42.16 mV. After modification, the surface charge of SiO2–NH2 was positive (ξ potential = +52.13 mV). It suggested that successful graft of APS onto the surface of SiO2 was achieved. Then, the positively charged SiO2–NH2 and negatively charged GO were mixed together and they assembled into a whole. To confirm the truth of the core–shell structure of SiO2@GO, the morphology and constituent elements were characterized, and the results are shown in the Fig. 2. | ||
| Fig. 2 Typical SEM images of (a) SiO2 and (b and c) SiO2@GO; (d) Raman spectra of GO and SiO2@GO; (e and f) EDX analysis of SiO2, SiO2–NH2, SiO2@GO and GO. | ||
According to Fig. 2(a), the surfaces of micron SiO2 particles are smooth. After modified by APS and encapsulated by GO sheets, one can clearly observe that there are some ultrathin GO sheets and folds firmly fixed on the surface of SiO2, as is shown in the Fig. 2(b) and (c). Raman spectroscopy is considered as a powerful tool to capture the presence of graphene and its derivatives. According to the Fig. 2(d), by means of Raman spectroscopy, the D and G band similar to these of original GO are observed at 1337 cm−1 and 1589 cm−1. It is known that GO disperses well in aqueous solution, even though exposed to the centrifugal environment of 5000 rpm min−1. So the existence of the characteristic peaks of GO indicates that the GO was not removed by centrifugation but attracted by SiO2–NH2 to form the core–shell structure. And it is interesting to find that the intensities of both the bands increases compared to these of GO under the same conditions characterized. We consider that the presence of GO on the surface of SiO2 results in the surface enhanced Raman effect (SERS), but the mechanism is still under study and the preliminary explanation is given as follows. Because GO absorbed on SiO2 could use the attached functional groups as nucleation centers.37,38 Based on the chemical mechanism of SERS, such electrostatic interaction could generate the charge-transfer complex to absorb light at the excitation frequency, resulting in chemical SERS. To further confirm the encapsulation of SiO2 particles by GO sheets and the effective grafting of APS, elemental analysis was also applied. As is shown in the Fig. 2(e) and (f), the relative atomic percentages of C, O and Si are respectively 6.73, 56.26 and 37.01. After being modified by APS, the relative atomic percentage of C obviously increases but the relative atomic percentages of O and Si decrease. Because for APS, the relative atomic percentages of Si and O are less than those of SiO2, but the relative atomic percentage of C is more than that of SiO2. So it suggests that APS was successfully grafted to SiO2. For SiO2@GO, the rise of C content from 12.32% of SiO2–NH2 to 21.55% also indicates the immobilization of GO on the SiO2.
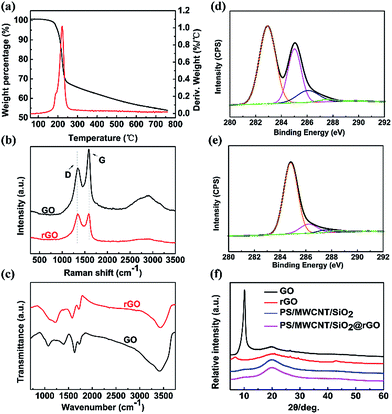
3.2 The reduction of GO
In order to determine the temperature for reducing GO, we firstly carried out the TGA experiment, as is shown in the Fig. 3(a). From the TGA results, it is observed that GO exhibits fast weight loss at about 170–250 °C, suggesting that abundant oxygen-containing functional groups can be removed above 170 °C. Hence, we prepared the PS nanocomposites above this temperature for 40 min to mildly reduce GO. FTIR spectra was utilized to discuss the degree of reduction from GO to rGO, and the results are shown in the Fig. 3(b). One can find that GO exhibits these characteristic peaks: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1730 cm−1), O–H (1622 cm−1), carboxyl C–O (1414 cm−1), epoxy C–O (1228 cm−1).39–41 After mild thermal reduction, it is obvious that the characteristic peaks for oxygen functional groups decrease. Especially, carboxyl C–O at 1414 cm−1 has disappeared. And the original aromatic C
O (1730 cm−1), O–H (1622 cm−1), carboxyl C–O (1414 cm−1), epoxy C–O (1228 cm−1).39–41 After mild thermal reduction, it is obvious that the characteristic peaks for oxygen functional groups decrease. Especially, carboxyl C–O at 1414 cm−1 has disappeared. And the original aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak at 1622 cm−1 shifts to 1577 cm−1, which is similar to the previous report.40 Furthermore, Raman spectra results plotted in the Fig. 3(c) show that the intensity ratio of the D and G band (ID/IG) changes from 0.684 to 0.974, suggesting the increase in average size of sp2 domain after thermal reduction.40 Similar phenomenon was observed by Paredes et al.,42 Kaner et al.43 and Stankovich et al.44 through chemical reduction. It was reported that reduction would cause the more aromatic domains of small overall size in rGO, leading to enhanced ID/IG ratio.44 Besides, the G band for rGO is shifted from 1589 cm−1 to 1586 cm−1, which is closer to that of pristine graphite, demonstrating the successfully reduction through this procedure. Moreover, XPS was also utilized to characterize the reduction of GO. According to XPS results, the C and O atomic percentage content were respectively 76.48% and 23.52%. The relatively high O content is ascribed to the rich oxygen-containing functional groups, according to the Fig. 3(d). After reduction, one can find that the oxygen peaks were obviously weakened (Fig. 3(e)), with the C and O atomic percentage content of 91.07% and 8.93%, indicating successfully reduction of GO. XRD is also an important tool to characterize the GO derivatives and its nanocomposites.45,46 Observed from the Fig. 3(f), the typical diffraction peak of GO was at 10.2°, corresponding to a certain interlayer spacing. After being reduced, this characteristic peak almost disappeared, indicating most of oxygen-containing functional groups were reduced. Moreover, no such peak was seen in PS/MWCNT/SiO2@rGO nanocomposites, which suggests that the periodic structure of GO disappeared and the sheets were exfoliated owing to absorption and uniform dispersion of SiO2@rGO.
C peak at 1622 cm−1 shifts to 1577 cm−1, which is similar to the previous report.40 Furthermore, Raman spectra results plotted in the Fig. 3(c) show that the intensity ratio of the D and G band (ID/IG) changes from 0.684 to 0.974, suggesting the increase in average size of sp2 domain after thermal reduction.40 Similar phenomenon was observed by Paredes et al.,42 Kaner et al.43 and Stankovich et al.44 through chemical reduction. It was reported that reduction would cause the more aromatic domains of small overall size in rGO, leading to enhanced ID/IG ratio.44 Besides, the G band for rGO is shifted from 1589 cm−1 to 1586 cm−1, which is closer to that of pristine graphite, demonstrating the successfully reduction through this procedure. Moreover, XPS was also utilized to characterize the reduction of GO. According to XPS results, the C and O atomic percentage content were respectively 76.48% and 23.52%. The relatively high O content is ascribed to the rich oxygen-containing functional groups, according to the Fig. 3(d). After reduction, one can find that the oxygen peaks were obviously weakened (Fig. 3(e)), with the C and O atomic percentage content of 91.07% and 8.93%, indicating successfully reduction of GO. XRD is also an important tool to characterize the GO derivatives and its nanocomposites.45,46 Observed from the Fig. 3(f), the typical diffraction peak of GO was at 10.2°, corresponding to a certain interlayer spacing. After being reduced, this characteristic peak almost disappeared, indicating most of oxygen-containing functional groups were reduced. Moreover, no such peak was seen in PS/MWCNT/SiO2@rGO nanocomposites, which suggests that the periodic structure of GO disappeared and the sheets were exfoliated owing to absorption and uniform dispersion of SiO2@rGO.
3.3 Electrical property of PS nanocomposites
PS/MWCNT nanocomposites have good electrical properties owing to excellent electrically conductive feature of MWCNT.47–51 It is well-known that introducing non-conductive particles into conductive fillers filled polymer may result in enhanced electrical conductivity due to volume exclusion effect. Moreover, mixing electrically conductive fillers with different geometric shapes and aspect ratio together in polymer matrix may lead to synergistic effect that will enhance the electrical conductivity of the complex. So we respectively combined SiO2 and this hybrid filler with PS and MWCNT. In order to study the difference of electrical conductivity between respectively introducing SiO2 and SiO2@rGO to PS/MWCNT, the DC electrical conductivity of the three types of PS nanocomposites is discussed as follows. According to the Fig. 4, the electrical conductivity of all the three types of PS nanocomposites increases as increase of MWCNT content. Before the percolation threshold, the upward trend of the electrical conductivity is not obvious. At the percolation threshold, a sharp increase of the electrical conductivity is observed according to the three curves, indicating an insulator–conductor transition at this time. After the percolation threshold, the upward trend tends to be gentle. Besides, one can find that the electrical conductivity of PS/MWCNT/SiO2 is relatively higher than that of PS/MWCNT especially near the percolation threshold. At the concentration of 2 wt%, increment in electrical conductivity with three orders of magnitude compared to that of PS/MWCNT can be observed. But at other concentrations, the enhancement is not obvious, suggesting that volume exclusion effect is not always work when the loading of non-conductive filler is not high enough. However, for PS/MWCNT/SiO2@rGO, the electrical conductivity is always much higher than that of PS/MWCNT/SiO2 or PS/MWCNT at the same content of MWCNT, particularly at the percolation threshold of MWCNT. One can observe that, at the MWCNT loading of 1.5 wt%, much higher electrical conductivity for PS/MWCNT/SiO2@rGO is obtained, presenting a jump with eight orders of magnitude compared to that of PS/MWCNT/SiO2 and PS/MWCNT. It suggests that the percolation threshold of PS/MWCNT/SiO2@rGO is largely reduced owing to the role of extremely few rGO coated on the surface of SiO2, and the mechanism will be discussed below.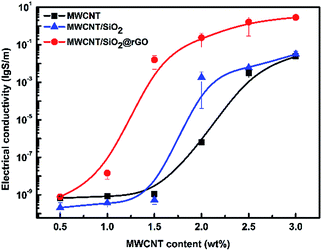 | ||
| Fig. 4 The electrical conductivity of PS/MWCNT, PS/MWCNT/SiO2 and PS/MWCNT/SiO2@rGO nanocomposites as increase of MWCNT. (The mass fractions of SiO2 and SiO2@rGO are all fixed at 20%.) | ||
3.4 Mechanism for enhanced electrical conductivity
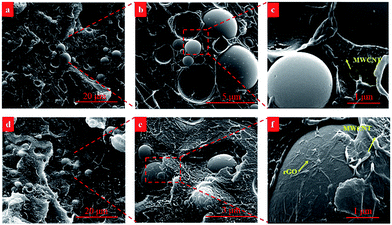
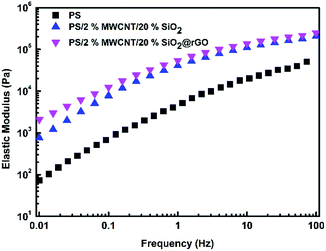
In order to truly observe the morphology and dispersed state of fillers to propose the mechanism, the cryo-fractured surfaces of PS/MWCNT/SiO2 and PS/MWCNT/SiO2@rGO nanocomposites were characterized by SEM, as is shown in the Fig. 5. According to the Fig. 5(a) and (b), one can observe that unmodified SiO2 particles whose surface is smooth disperse relatively homogeneous in PS matrix. And these large SiO2 particles can exclude MWCNT from their dominant areas, so MWCNT can connect with each other in a higher probability. As consequence, the effective concentration of MWCNT is increased (see the Fig. 5(c)). For PS/MWCNT/SiO2@rGO, the dispersed state of both MWCNT and SiO2@rGO is similar to that of PS/MWCNT/SiO2. Because PS/MWCNT/SiO2 and PS/MWCNT/SiO2@GO nanocomposites were prepared in the DMF solution via intense mechanical stirring. When they were precipitated in deionized water, the dispersed state of SiO2 and SiO2@GO in PS matrix was fixed. Hence, when these nanocomposites were compressed and reduced at high temperature, the filler dispersion of SiO2 and SiO2@rGO in PS matrix was similar although the interaction between rGO and PS is good. However, from the Fig. 5(e) and (f), one can find that the conductive rGO folds fixed firmly on the surface of SiO2 are connected well with MWCNT as due to π–π interactions, and this conductive rGO shell make non-conductive SiO2 become conductive particles through transforming electrons by surficial immobilized rGO. So we deem that this would result in not only increased effective concentration of MWCNT owing to volume exclusion effect of SiO2@rGO, but also improved conductive network owing to synergistic effect.Rheological properties of molten composites are considered to be effective to reflect the filler dispersion state. Since SEM observation demonstrated that the dispersed state of the fillers for both PS/MWCNT/SiO2 and PS/MWCNT/SiO2@rGO are similar, in order to prove this observation further, the melt rheology of PS nanocomposites was measured and the results were plotted in the Fig. 6. In melt rheology test, PS nanocomposites filled with 2 wt% MWCNT were randomly selected as the example. Clearly the composites modulus are higher than that of neat PS. Specifically, PS/2% MWCNT/SiO2@rGO exhibits comparative elastic modulus in comparison with that of PS/2% MWCNT/SiO2, indicating that unchanged dispersed state of SiO2 and MWCNT in PS matrix after encapsulation by rGO. So the largely enhanced electrical conductivity and obviously decreased percolation threshold are ascribed to both volume exclusion and synergistic effects discussed below instead of improved dispersion for fillers.
To vividly explain the mechanism concluded from the discussions above, a schematic of fillers' dispersed state in PS nanocomposites is plotted in the Fig. 7. For MWCNT uniformly dispersing in PS, conductive network needs a certain amount of fillers which are so close that can meet with electron transition. Hence the electrons can pass through the composites by the MWCNT network. Once vast non-conductive SiO2 particles are added, these large-size granules will exclude MWCNT from their dominant regions, leading to increased effective concentration of MWCNT that form conductive network in the segregated areas. According to the previous report,25 the effective concentration of MWCNT is defined as VMWCNT/(VMWCNT + Vpolymer). Hence, for example, when only 20 wt% SiO2 was added, the effective concentration of MWCNT increased by 9.5% at the MWCNT loading of 1.5 wt%, resulting in enhancement with orders of magnitude in electrical conductivity near the percolation threshold. However, after the percolation, the increased effective concentration is limited, thus leading to inconspicuous enhancement in electrical properties compared to that of PS/MWCNT. But for PS/MWCNT/SiO2@rGO, although encapsulation by rGO has not changed the dispersion trait entirely, SiO2@rGO can be considered as electrically conductive particles which conduct electrons through rGO on the surface of insulating SiO2. Moreover, different from poor interactions and separation between unmodified SiO2 and MWCNT, the rGO coated on the surface of particles make them connect well with MWCNT due to π–π interactions. As a result, not only do these SiO2@rGO isolate MWCNT from their occupied space on account of volume exclusion effect, but also link the gaps present between the unconnected MWCNT to perfect the conductive network. This is mainly ascribed to different geometric shapes and aspect ratio as well as dispersion features between SiO2@rGO and MWCNT. Hence, the electrical conductivity of PS/MWCNT/SiO2@rGO demonstrated a largely enhanced result.
 | ||
| Fig. 7 Schematic of fillers' dispersed state in PS nanocomposites: (a) PS/MWCNT; (b) PS/MWCNT/SiO2; (c) PS/MWCNT/SiO2@rGO. | ||
3.5 Electromagnetic interference (EMI) shielding effectiveness
Owing to the obvious increase in electrical conductivity of PS/MWCNT/SiO2@rGO nanocomposites, this prepared material is considered to possess potential application in EMI shielding.10,52 For this reason, the EMI shielding effectiveness (SE) was studied and this was plotted in the Fig. 8. One can see that for all the three types of PS nanocomposites, the EMI shielding effectiveness increases as increase of MWCNT content. The shielding effectiveness of PS/MWCNT/SiO2 with 2 wt% MWCNT loading shows shielding effectiveness of 13.2–21.9 dB over the frequency of 8–12 GHz. Further raising MWCNT content to 3 wt%, the SE of PS/MWCNT/SiO2 increases to 13.8–27.1 dB, which is quite with that of PS/MWCNT because of comparative electrical conductivity. Furthermore, one can find that the EMI shielding effectiveness of PS/MWCNT or PS/MWCNT/SiO2 is sensitive to frequency. However, for PS/MWCNT/SiO2@rGO, the shielding effectiveness is almost independent of frequency in the measured frequency region. And the shielding effectiveness is relatively higher at the same frequency, especially at the low frequency. For example, at the MWCNT content of 3 wt%, the EMI shielding effectiveness of PS/MWCNT/SiO2@rGO increased 16.5 dB (almost 120%) at 8 GHz compared to that of PS/MWCNT/SiO2. This largely improved EMI property is mostly ascribed to more complete conductive network and higher electrical conductivity of PS/MWCNT/SiO2@rGO. According to previous study, this high electrical conductivity and good EMI properties are comparative with those of PS/graphene composites at the same filler concentration.53–56 However, why the frequency-dependence for PS/MWCNT/SiO2@rGO is different from that of PS/MWCNT or PS/MWCNT/SiO2 is not understood and this will be studied in the future.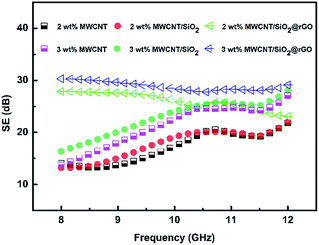 | ||
| Fig. 8 Electromagnetic interference shielding effectiveness of PS/MWCNT, PS/MWCNT/SiO2 and PS/MWCNT/SiO2@rGO nanocomposites as a function of frequency measured in 8–12 GHz range. | ||
3.6 Thermal stability
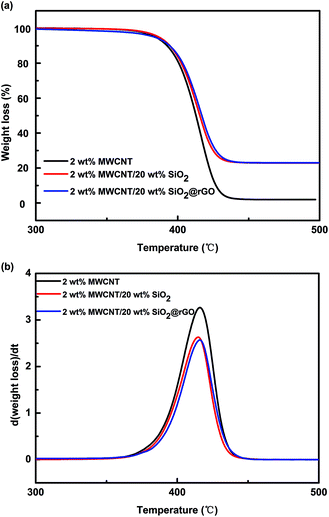
Thermal stability is important for PS composites no matter in melt processing or high temperature application. Hence, TGA study was used to investigate the effects of different fillers on the thermal stability of PS nanocomposites, and the results were plotted in the Fig. 9. Except for the adsorbed water, there is almost no mass loss at the temperature lower than 350 °C. According to the Fig. 9(a), one can judge that the SiO2 or SiO2@rGO loading is 20 wt%, and the content of rGO is scarcely (≤0.2 wt%). Obtained from the Fig. 9(b), PS/MWCNT decomposes rapidly at 415.8 °C, and the presence of 20 wt% SiO2 in the PS/MWCNT nanocomposites reduce the thermal stability, leading to the reduction of the maximum decomposition temperature (414.6 °C). After the SiO2 being encapsulated by rGO, the maximum decomposition temperature increases to 416.2 °C. However, the difference is not great. It suggests that either adding SiO2 or introducing SiO2@rGO to PS/MWCNT, the thermal stability of PS nanocomposites are well maintained.4. Conclusion
The core–shell structure of SiO2@GO has been successfully prepared by a simple solution blending method. And the application of this hybrid filler in PS/MWCNT demonstrates the enhanced electrical conductivity and EMI shielding effectiveness. We attribute the enhancement to the volume exclusion effect of SiO2@rGO as well as synergistic effect between SiO2@rGO and MWCNT. And we believe that our study will provide a better understanding of volume exclusion effect and synergistic effect for the enhancement of electrical property.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51421061 and 51210005). We would like to express our great thanks to Guangdong Shengyi Technology Limited Corporation for financial support.Notes and references
- H. Deng, T. Skipa, E. Bilotti, R. Zhang, D. Lellinger and L. Mezzo, et al., Adv. Funct. Mater., 2010, 20, 1424–1432 CrossRef CAS.
- H. Deng, L. Lin, M. Ji, S. Zhang, M. Yang and Q. Fu, Prog. Polym. Sci., 2014, 39, 627–655 CrossRef CAS.
- S. Maiti, N. K. Shrivastava, S. Suin and B. B. Khatua, ACS Appl. Mater. Interfaces, 2013, 5, 4712–4724 CAS.
- V. Eswaraiah, K. Balasubramaniam and S. Ramaprabhu, J. Mater. Chem., 2011, 21, 12626–12628 RSC.
- T. Sekitani, Y. Noguchi, K. Hata, T. Fukushima, T. Aida and T. Someya, Science, 2008, 321, 1468–1472 CrossRef CAS PubMed.
- C. A. Hewitt, A. B. Kaiser, S. Roth, M. Craps, R. Czerw and D. L. Carroll, Nano Lett., 2012, 12, 1307–1310 CrossRef CAS PubMed.
- M. H. Al-Saleh and U. Sundararaj, Carbon, 2009, 47, 1738–1746 CrossRef CAS.
- S. P. Pawar, K. Pattabhi and S. Bose, RSC Adv., 2014, 4, 18842–18852 RSC.
- Y. Chen, Y. Li, D. Xu and W. Zhai, RSC Adv., 2015, 5, 82034–82041 RSC.
- Y. Chen, H. B. Zhang, Y. Yang, M. Wang, A. Cao and Z. Z. Yu, Adv. Funct. Mater., 2016, 26, 447–455 CrossRef CAS.
- Y. Chen, Y. Wang, H.-B. Zhang, X. Li, C.-X. Gui and Z.-Z. Yu, Carbon, 2015, 82, 67–76 CrossRef CAS.
- Y. Chen, H.-B. Zhang, Y. Huang, Y. Jiang, W.-G. Zheng and Z.-Z. Yu, Compos. Sci. Technol., 2015, 118, 178–185 CrossRef CAS.
- H.-B. Zhang, Q. Yan, W.-G. Zheng, Z. He and Z.-Z. Yu, ACS Appl. Mater. Interfaces, 2011, 3, 918–924 CAS.
- A.-C. Baudouin, J. Devaux and C. Bailly, Polymer, 2010, 51, 1341–1354 CrossRef CAS.
- A. Göldel, G. Kasaliwal and P. Pötschke, Macromol. Rapid Commun., 2009, 30, 423–429 CrossRef PubMed.
- C. Zhang, L. Wang, J. Wang and C.-a. Ma, Carbon, 2008, 46, 2053–2058 CrossRef CAS.
- I. Alig, P. Pötschke, D. Lellinger, T. Skipa, S. Pegel and G. R. Kasaliwal, et al., Polymer, 2012, 53, 4–28 CrossRef CAS.
- D. Lellinger, D. Xu, A. Ohneiser, T. Skipa and I. Alig, Phys. Status Solidi B, 2008, 245, 2268–2271 CrossRef CAS.
- E. N. J. Ford, M. L. Minusa, T. Liu, J. I. Choi, S. S. Jang and S. Kumar, Macromol. Chem. Phys., 2012, 213, 617–626 CrossRef.
- S. M. Miriyala, Y. S. Kim, L. Liu and J. C. Grunlan, Macromol. Chem. Phys., 2008, 209, 2399–2409 CrossRef CAS.
- I. Jurewicz, P. Worajittiphon, A. A. King, P. J. Sellin, J. L. Keddie and A. B. Dalton, J. Phys. Chem. B, 2011, 115, 6395–6400 CrossRef CAS PubMed.
- B. W. Steinert and D. R. Dean, Polymer, 2009, 50, 898–904 CrossRef CAS.
- H. Pang, C. Chen, Y.-C. Zhang, P.-G. Ren, D.-X. Yan and Z.-M. Li, Carbon, 2011, 49, 1980–1988 CrossRef CAS.
- M. Kotaki, K. Wang, M. L. Toh, L. Chen, S. Y. Wong and C. He, Macromolecules, 2006, 39, 908–911 CrossRef CAS.
- H.-D. Bao, Z.-X. Guo and J. Yu, Polymer, 2008, 49, 3826–3831 CrossRef CAS.
- W. Zhang, R. S. Blackburn and A. A. Dehghani-Sanij, Scr. Mater., 2007, 56, 581–584 CrossRef CAS.
- S. Zhang, L. Lin, H. Deng, X. Gao, E. Bilotti and T. Peijs, et al., eXPRESS Polym. Lett., 2012, 6, 159–168 CrossRef CAS.
- M. Safdari and M. S. Al-Haik, Carbon, 2013, 64, 111–121 CrossRef CAS.
- K.-S. Kim and S.-J. Park, Carbon Letters, 2012, 13, 51–55 CrossRef.
- Z.-Y. Xiong, B.-Y. Zhang, L. Wang, J. Yu and Z.-X. Guo, Carbon, 2014, 70, 233–240 CrossRef CAS.
- M.-C. Hermant, P. van der Schoot, B. Klumperman and C. E. Koning, ACS Nano, 2010, 4, 2242–2248 CrossRef CAS PubMed.
- S. Yang, X. Feng, S. Ivanovici and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 8408–8411 CrossRef CAS PubMed.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- L. Chen, S. Chai, K. Liu, N. Ning, J. Gao and Q. Liu, et al., ACS Appl. Mater. Interfaces, 2012, 4, 4398–4404 CAS.
- J. L. Vickery, A. J. Patil and S. Mann, Adv. Mater., 2009, 21, 2180–2184 CrossRef CAS.
- T. H. Han, W. J. Lee, D. H. Lee, J. E. Kim, E. Y. Choi and S. O. Kim, Adv. Mater., 2010, 22, 2060–2064 CrossRef CAS PubMed.
- Y.-C. Chen, R. J. Young, J. V. Macpherson and N. R. Wilson, J. Phys. Chem. C, 2007, 111, 16167–16173 CAS.
- C. Xu and X. Wang, Small, 2009, 5, 2212–2217 CrossRef CAS PubMed.
- A. Suspension, Characterization of chemically modified graphene sheets park, Sungjin, 2008 Search PubMed.
- W. Chen, L. Yan and P. R. Bangal, Carbon, 2010, 48, 1146–1152 CrossRef CAS.
- P. K. S. Mural, M. Sharma, G. Madras and S. Bose, RSC Adv., 2015, 5, 32078–32087 RSC.
- J. Paredes, S. Villar-Rodil, P. Solis-Fernandez, A. Martinez-Alonso and J. Tascon, Langmuir, 2009, 25, 5957–5968 CrossRef CAS PubMed.
- V. C. Tung, M. J. Allen, Y. Yang and R. B. Kaner, Nat. Nanotechnol., 2009, 4, 25–29 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes and Y. Jia, et al., Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- J. Liang, Y. Huang, L. Zhang, Y. Wang, Y. Ma and T. Guo, et al., Adv. Funct. Mater., 2009, 19, 2297–2302 CrossRef CAS.
- X. Du, Z.-Z. Yu, A. Dasari, J. Ma, M. Mo and Y. Meng, et al., Chem. Mater., 2008, 20, 2066–2068 CrossRef CAS.
- M. Mahmoodi, M. Arjmand, U. Sundararaj and S. Park, Carbon, 2012, 50, 1455–1464 CrossRef CAS.
- A. K. Kota, B. H. Cipriano, M. K. Duesterberg, A. L. Gershon, D. Powell and S. R. Raghavan, et al., Macromolecules, 2007, 40, 7400–7406 CrossRef CAS.
- G. Sun, G. Chen, Z. Liu and M. Chen, Carbon, 2010, 48, 1434–1440 CrossRef CAS.
- H. Peng and X. Sun, Chem. Phys. Lett., 2009, 471, 103–105 CrossRef CAS.
- Y. Yang, M. Gupta, J. Zalameda and W. Winfree, Micro Nano Lett., 2008, 3, 35–40 CAS.
- H.-B. Zhang, W.-G. Zheng, Q. Yan, Z.-G. Jiang and Z.-Z. Yu, Carbon, 2012, 50, 5117–5125 CrossRef CAS.
- X.-Y. Qi, D. Yan, Z. Jiang, Y.-K. Cao, Z.-Z. Yu and F. Yavari, et al., ACS Appl. Mater. Interfaces, 2011, 3, 3130–3133 CAS.
- W. Li, X.-Z. Tang, H.-B. Zhang, Z.-G. Jiang, Z.-Z. Yu and X.-S. Du, et al., Carbon, 2011, 49, 4724–4730 CrossRef CAS.
- D.-X. Yan, P.-G. Ren, H. Pang, Q. Fu, M.-B. Yang and Z.-M. Li, J. Mater. Chem., 2012, 22, 18772–18774 RSC.
- D. X. Yan, H. Pang, B. Li, R. Vajtai, L. Xu and P. G. Ren, et al., Adv. Funct. Mater., 2015, 25, 559–566 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |