Enhancement of Ce1−xSnxO2 support in LaMnO3 for the catalytic oxidation and adsorption of elemental mercury†
Haomiao Xua,
Yongpeng Mab,
Songjian Zhaoa,
Wenjun Huanga,
Zan Qua and
Naiqiang Yan*a
aSchool of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: nqyan@sjtu.edu.cn; Fax: +86 21 54745591; Tel: +86 21 54745591
bHenan Collaborative Innovation Center of Environmental Pollution Control and Ecological Restoration, Zhengzhou University of Light Industry, No. 136, Science Avenue, Zhengzhou 450001, China
First published on 8th June 2016
Abstract
Mn-based perovskite oxide was used as the active site for elemental mercury (Hg0) removal from coal-fired flue gas. Ce1−xSnxO2 binary oxides were selected as the catalyst supports for LaMnO3 to enhance the catalytic oxidation and adsorption performance. Ce0.7Sn0.3O2 had the best Hg0 removal performance among the as-prepared Ce1−xSnxO2 binary oxides; the Hg0 removal efficiency was 95.2% at 350 °C. LaMnO3 had better performance at low temperatures (<200 °C). LaMnO3/Ce0.7Sn0.3O2 enlarged the reaction temperature window and enhanced the Hg0 removal efficiencies. The correlation between the physicochemical properties and the catalytic removal performance was investigated by XRD, BET surface area measurements, Raman spectroscopy, H2-TPR and XPS analysis. With the addition of Ce–Sn binary oxides as catalyst support, the surface areas of LaMnO3 was enlarged, the reducibility was enhanced and the oxygen mobility was improved. In addition, the Hg0 removal mechanism was illustrated on the basis of the experimental results. The roles of Ce, Sn and LaMnO3 were also discussed in this study.
1. Introduction
Elemental mercury (Hg0) emitted from coal-fired power plants is hazardous pollutant in the atmosphere.1–3 Hg0 is hardly removed due to its insolubility in water and high volatility.4 Currently, the efficient methods for the removal of Hg0 make full use of the existing air pollution control devices (APCDs) to remove Hg0 and NOx/particles/SO2.2,5 In general, catalytic oxidation and adsorption are two primary mechanisms for Hg0 removal. Hg0 transformed to oxidized state (Hg2+) by catalytic oxidation could be easily removed in the wet flue gas desulfurization (WFGD) system. Hg0 changed to particle-bound mercury (Hgp) can be captured by dust-cleaning apparatus. In the dust cleaning process, the catalytic oxidation of Hg0 to Hg2+ was also beneficial for chemical adsorption. Therefore, catalytic oxidation of Hg0 to Hg2+ was the key to controlling Hg0 emission.Among the catalysts or sorbents in recent studies, Mn-based metal oxides were proven to be efficient materials for Hg0 removal from coal-fired flue gas.6–12 The high redox potential makes them possible for Hg0 oxidation to Hg2+, along with the adsorption on the surface of materials. In this process, higher catalytic performance and sufficient surface oxygen were beneficial for Hg0 removal. However, Mn-based metal oxides often suffer two problems for Hg0 removal: (1) particle aggregation resulted in the low stability, and it is hard to make full use of MnOx particles; and (2) MnOx often loses its high activity when the reaction temperature is higher than >200 °C. To solve the first problem, perovskite oxides attracted our attention due to their excellent catalytic oxidation performance. Higher dispersion of Mn ions occupies the B sites in ABO3 perovskite structure.13–15 LaMnO3 indicated high Hg0 removal performance in our recent study. However, it also suffers from low activity at high temperature. In our previous studies, doping of SnO2 was employed to enhance the Hg0 removal performance at high temperature.7,16 Sn–Mn binary metal oxides enlarged the reaction temperature window. In fact, SnO2 had excellent electron transfer and O2 capture performance.17,18 O2 in the gas can be adsorbed on the surface of SnO2. Then, the adsorbed O2 changed to O2− by SnO2 offering electrons on its surface. The sufficient O2− was favorable for Hg2+ capture. In addition, CeO2 has large oxygen storage capacity and oxygen conversion ability. Ce–Mn mixed metal oxides may have high catalytic oxidation performance because it makes good use of O2 in the simulated flue gas.19 Liu et al. reported that doping with Ce into SnO2 increased the surface area, decreased the crystallite sizes, and it showed higher catalytic activity for the catalytic combustion of methane.18 It was interesting to determine whether Ce–Sn binary metal oxides can be used for Hg0 catalytic oxidation.
In view of the complete perovskite structure, LaMnO3 had higher catalytic performance. Ce–Sn binary oxides were impossible to dope into the perovskite crystal lattice. To date, few reports have discussed the Ce–Sn binary metal oxides used as catalyst supports. In this study, we first optimized the Ce–Mn binary oxides for Hg0 removal. Subsequently, the Ce–Mn binary oxides were used as a catalyst support for LaMnO3. The performances for Hg0 removal over these as-prepared materials were evaluated in a fixed-bed adsorption system. The roles of LaMnO3 and the Ce–Sn support were discussed in our study. On this basis, the relationship between the active center and catalyst support was also discussed by physico-chemical characterization. The possible Hg0 removal mechanism, as well as the application prospect, was proposed accordingly.
2. Experimental section
2.1 Materials preparation and characterization
Ce1−xSnxO2 (x = 0.1, 0.3, 0.5, 0.7, and 0.9) supports were prepared by a co-precipitation method. Suitable amounts of Ce(NO3)4 and SnCl4 dissolved in distilled water. A stoichiometric amount of ammonia was added to the mixture as the precipitation agent under strong stirring for 2 h. The precipitate was then filtered and washed with deionized water three times to remove Cl− from the water. Lastly, the precipitate was transferred to a muffle furnace and calcined at 500 °C for 5 h. All the samples used as catalysts were ground to a 40–60 mesh size.Ce1−xSnxO2 was selected as a catalyst support for LaMnO3. The prepared Ce1−xSnxO2 supports were ground to <100 mesh size. The LaMnO3/Ce1−xSnxO2 was prepared using a sol–gel method. The required amount of La(NO3)3 and Mn(NO3)3 was dissolved together in Ce1−xSnxO2 solution, followed by addition of citric acid (CA) in the mixed solution. The temperature of the aqueous solution was maintained constant at 80 °C. The molar ratio for each component was La/Mn/CA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. After vigorous stirring and evaporation, a transparent gel was formed, which was then dried at 90 °C overnight. The obtained precursor was first calcined at 400 °C for 1 h in air to decompose citric acid totally and was then calcined at 750 °C for 5 h with a rate of 10 °C min−1. All the samples were grounded to 40–60 mesh.
2. After vigorous stirring and evaporation, a transparent gel was formed, which was then dried at 90 °C overnight. The obtained precursor was first calcined at 400 °C for 1 h in air to decompose citric acid totally and was then calcined at 750 °C for 5 h with a rate of 10 °C min−1. All the samples were grounded to 40–60 mesh.
The as-prepared materials were characterized by means of BET surface area measurements, XRD, Raman spectroscopy, H2-TPR and XPS. The detail analysis can be found in ESI.†
2.2 Activities test
The performance for Hg0 removal over the as-prepared materials was evaluated using a fixed-bed reactor. A Hg0 permeation tube was used to generate Hg0 vapor carried by pure N2, which was introduced to the inlet of the gas mixer. Other gases such as O2 were introduced to the gas mixer at constant flows. The mass flow rate was controlled by mass flow controllers (MFC). A fixed-bed reactor system was used to investigate the Hg0 adsorption performance. The reaction temperature was controlled from 100 to 350 °C using a temperature controller tubular furnace. In each test, the as-prepared materials were placed into the reaction tube, which was placed to control the reaction temperature. The cold vapor atomic absorption spectroscopy (CVASS) analyzer was used as the online continuous detector. The concentration of Hg0 was calculated by Lumex RA 915+. The inlet concentration of Hg0 was 500 ± 50 μg m−3. At the beginning of each test, the simulated gas bypassed the reactor and the inlet gas was detected to ensure a stable Hg0 concentration. The simulated gas passed the samples and the Hg0 concentration was detected by CVASS online. The mass of the material for each test was 30 mg.The Hg0 removal efficiency was calculated according to eqn (1):
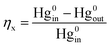 | (1) |
2.3 Hg-TPD method
Mercury temperature programmed desorption (Hg-TPD) method was built to evaluate the desorption performance of the as-prepared materials. Before each test, the sorbents were first under adsorption for 20 min at 150 °C with 4% O2 balanced with N2 (total flow rate = 500 mL min−1). After the furnace was cooled to 100 °C, the materials were regenerated by heating from 100 °C to 700 in a pure N2 carrier gas. The heating rate was set as 5 °C min−1. The mercury signal was recorded by CVASS online system.3. Results & discussion
3.1 Hg0 removal performance
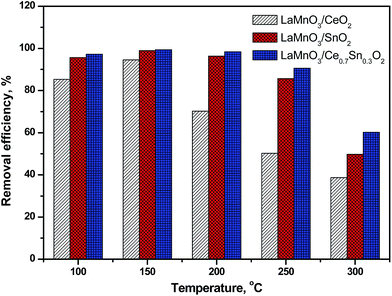
To further identify the effects of the Ce0.7Sn0.3O2 catalyst support for LaMnO3 on the Hg0 removal efficiency, the performances of LaMnO3/CeO2, LaMnO3/SnO2 and LaMnO3/Ce0.7Sn0.3O2 were evaluated at a wide temperature window. As shown in Fig. 3, LaMnO3/CeO2 had the highest Hg0 removal efficiency (94.5%) at 150 °C, while the removal efficiency decreased to only 38.5% when the temperature was increased to 300 °C. Higher temperatures resulted in low activity over Mn-based material. However, when SnO2 acted as catalyst support for LaMnO3, the Hg0 removal efficiency increased, especially at high temperatures (>200 °C). The Hg0 removal efficiency was 85.63% at 250 °C, which was higher than that compared with 50.3% of LaMnO3/CeO2 at the same temperature. For LaMnO3/Ce0.7Sn0.3O2, the performance was further enhanced compared to that of LaMnO3/SnO2. The Hg0 removal efficiency was greater than 90% even when the temperature was as high as 250 °C, and the Hg0 removal efficiency can maintain about 60% at 300 °C. These results indicated that SnO2 support can enlarge the reaction temperature window and Ce0.7Sn0.3O2 acted as LaMnO3's support showed excellent Hg0 removal performance.
3.2 Characterization of the materials
X-ray power diffraction (XRD) was utilized to clarify the crystal phases of the as-prepared Ce1−xSnxO2 binary metal oxides and their supported material. As depicted in Fig. 5(a), it showed the XRD patterns of Ce1−xSnxO2. For SnO2, the diffraction peaks of can be well indexed to SnO2 (JPCDS no. 21-1250). The diffraction peaks of CeO2 were ascribed to CeO2 (JPCDS no. 43-1002). With the addition of CeO2 in SnO2, the Ce0.1Sn0.9O2 had the CeO2 phase. However, there were no SnO2 phases for Ce0.9Sn0.1O2 binary oxides. It could be speculated that the Sn was dispersed on the CeO2 surface and/or incorporated into CeO2 lattice.18 As for the Sn-rich materials, the XRD patterns of the Ce0.1Sn0.9O2 and Ce0.3Sn0.7O2, the patterns existed as a mixed form of CeO2 and SnO2. Even for the Ce0.5Sn0.5O2 and Ce0.7SnO0.3O2, the mixing phase co-existed, but the intensity of the SnO2 phase was not as strong as CeO2. It could be speculated that, for Ce0.7Sn0.3O2, some of the SnO2 entered into the lattice of CeO2 and some of SnO2 existed on the surface of the mixed metal oxides. Furthermore, as shown in Fig. 5(b), the XRD patterns of LaMnO3/CeO2, LaMnO3/CeO2 and LaMnO3/Ce0.7Sn0.3O2 were also represented. The peaks at 22.8, 32.6, 40.2, 46.7, 52.8, 58.1, 68.3 and 77.8° 2θ, all the characteristic peaks were indexed to a perovskite phase (PDF-88-0633). For LaMnO3/CeO2, the primary crystal was the perovskite structure. Some peaks can be ascribed to CeO2.23 No other peaks were generated on the composite surface. Similarly, the peaks of LaMnO3/SnO2 had the crystal phase of perovskite oxide and SnO2. For LaMnO3/Ce0.7Sn0.3O2, perovskite oxide phase was also the primary crystal structure, LaMnO3 and Ce0.7Sn0.3O2 maintained their respective crystal phase. | ||
| Fig. 5 XRD patterns of (a) Ce1−xSnxO2 mixed oxides, and (b) LaMnO3/SnO2, LaMnO3/Ce0.7Sn0.3O2 and LaMnO3/CeO2. | ||
BET analysis was conducted for the as-prepared samples. The BET surface area and the BJH total pore volume are listed in Table 1. Pure LaMnO3 had a surface area of 16.60 m2 g−1, which is lower than pure SnO2 (29.89 m2 g−1) and CeO2 (46.32 m2 g−1). For Ce0.7Sn0.3O2, the surface area was 35.74 m2, which was larger than SnO2 but smaller than CeO2. The addition of SnO2 to CeO2 increased the pore volume, resulting in a larger surface area. Similarly, the surface area of LaMnO3/SnO2 (21.77 m2) was smaller than LaMnO3/CeO2 (66.35 m2). However, when Ce0.7Sn0.3O2 acted as the catalyst support, MnOx/Ce0.7Sn0.3O2 had surface areas of only 4.37 m2 and a very small pore volume (0.056 m3). This was the reason why the MnOx particles was easily aggregated.8 LaOx/Ce0.7Sn0.3O2 had a larger surface area of 74.21 m2 and the larger pore volume of 0.625 m3. LaOx can form porous structures combined with Ce–Sn binary oxides. For LaMnO3/Ce0.7Sn0.3O2, it had the largest surface area (86.08 m2) among the as-prepared materials. Mn-based oxides often suffer from the problem of particle aggregation. CeO2 can enlarge the surface area of MnOx and SnO2 particles addition could make a larger pore volume, resulting in a larger surface area. The larger surface area was favorable for the Hg0 reaction on its surface. According to XRD analysis, the LaMnO3 perovskite oxide and Ce–Sn binary oxide maintained had their own crystal structure. The cooperation of them benefited the building the larger pore and the dispersion of Mn-based material.
| Materials | BET surface area (m2 g−1) | BJH pore volume (m3) |
|---|---|---|
| LaMnO3 | 16.60 | 0.118 |
| SnO2 | 29.89 | 0.141 |
| CeO2 | 46.32 | 0.130 |
| Ce0.7Sn0.3O2 | 35.74 | 0.152 |
| LaOx/Ce0.7Sn0.3O2 | 74.21 | 0.625 |
| MnOx/Ce0.7Sn0.3O2 | 4.37 | 0.056 |
| LaMnO3/SnO2 | 21.77 | 0.201 |
| LaMnO3/CeO2 | 66.35 | 0.302 |
| LaMnO3/Ce0.7Sn0.3O2 | 86.08 | 0.434 |
To reveal the effect of Ce–Sn binary oxide support on LaMnO3, Raman spectroscopy technique was used for structure analysis. For comparison, LaOx/Ce0.7Sn0.3O2 and MnOx/Ce0.7Sn0.3O2 were also collected. As shown in Fig. 6, a wide peak at about 500–750 cm−1 was observed for LaMnO3/SnO2. This was attributed to the surface vibrational mode of Sn–O–Sn (A1g) in nanocrystalline SnO2 and to volume modes of SnO2.24 The peak at about 648 cm−1 was ascribed to the overlap of MnO2 with SnO2.25 There were no obvious peaks in the LaMnO3/CeO2 spectrum under the same intensity. With the addition of Sn to the CeO2 support, the A1g peak widened and vanished due to the presence of bulk oxygen vacancies, resulting in a distortion of the Sn–O bond.18 The peak at about 461, 462 or 468 cm−1 can be ascribed to the F2g vibration mode of CeO2 in the fluorite structure, the slight shift from 461 to 468 cm−1 in the F2g mode was due to nanosize sample.26 Obviously, MnO2 overlapped on SnO2 from the spectrum of LaMnO3/Ce0.7Sn0.3O2, the addition of CeO2 increased more oxygen vacancies.17 The addition of Sn enhanced the role of MnO2 which was the main active site for Hg0 catalytic oxidation. Significantly, the addition of Ce to catalyst support generated more oxygen vacancies, which could enhance the redox ability of the material, resulting in the higher catalytic performance.
 | ||
| Fig. 6 Raman spectra of LaOx/Ce0.7Sn0.3O2, MnOx/Ce0.7Sn0.3O2, LaMnO3/Ce0.7Sn0.3O2, LaMnO3/SnO2, and LaMnO3/CeO2. | ||
The reducibility of the samples was assessed by H2-TPR. Fig. 7 shows the qualitative profiles recorded over the as-prepared materials. For LaOx/Ce0.7Sn0.3O2, it presented a wide peak centered at about 531.1 °C. In general, Sn4+ can be only reduced to Sn2+ below 500 °C, and Sn2+ reduced to Sn0 at the temperature higher than 500 °C. The shoulder peak over LaOx/Ce0.7Sn0.3O2 could indicate the role of Ce, the reducibility of Ce4+ and Sn4+ could be promoted simultaneously according to the equilibrium of Sn4+ + 2Ce3+ ↔ Sn2+ + 2Ce4+.18 MnOx/Ce0.7Sn0.3O2 presented two character peaks at 488.9 and 595.6 °C, which were assigned to Mn4+ → Mn3+ and Mn3+ → Mn2+, respectively.27 In the LaMnO3/SnO2 profile, there were four peaks at 325.2, 547.5, 632.3 and 799.5 °C. The reduction peak of Mn4+ → Mn3+ moved to a lower temperature (325.2 °C). It was speculated that the SnO2 support was beneficial for the redox of Sn2+ + Mn4+ ↔ Sn4+ + Mn3+. In addition, the peak of Mn3+ → Mn2+ moved to a higher temperature compared to MnOx/Ce0.7Sn0.3O2. However, with the addition of Ce to the SnO2 support, for LaMnO3/CeO2, a wide shoulder peak contributed to Mn4+ → Mn3+ → Mn2+ at temperatures lower than 500 °C. Moreover, in the spectrum of LaMnO3/Ce0.7Sn0.3O2, two peaks at 344.8 and 530.4 °C can be ascribed to Mn4+ → Mn3+ and Mn3+ → Mn2+, respectively.28 The reduction of Mn4+ → Mn3+ was beneficial for Hg0 oxidation. Ce–Sn binary oxides improved the reducibility compared with MnOx/Ce0.7Sn0.3O2.
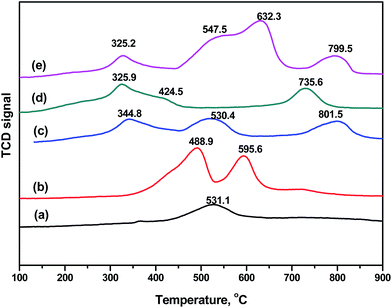 | ||
| Fig. 7 H2-TPR profile of the as-prepared materials: (a) LaOx/Ce0.7Sn0.3O2, (b) MnOx/Ce0.7Sn0.3O2 and (c) LaMnO3/Ce0.7Sn0.3O2, (d) LaMnO3/CeO2, (e) LaMnO3/SnO2. | ||
Based on the H2-TPR results, it indicated that the perovskite structure (LaMnO3/Ce0.7Sn0.3O2) showed higher reducibility than pure MnOx/Ce0.7Sn0.3O2, which was beneficial for catalytic oxidation. Furthermore, Sn2+ can easily offer electrons, which also can enhance the reducibility of Mn4+ to Mn3+. The addition of Ce enhanced the reducibility of SnO2 and MnOx. It was speculated that the reduction of Mn4+ → Mn3+ → Mn2+ can be enhanced by Sn2+ → Sn4+ or Ce2+ → Ce3+ → Ce4+. CeO2 and SnO2 can also interact with each other. The interaction among the catalytic support and active sites resulted in high catalytic oxidation performance.
To discuss the Hg0 removal mechanism over LaMnO3/Ce0.7Sn0.3O2, the XPS spectra of O, Mn and Hg over the fresh and after adsorption of LaMnO3/Ce0.7Sn0.3O2 are shown in Fig. 8. For the fresh LaMnO3/Ce0.7Sn0.3O2 sample, Fig. 8(a) shows two peaks of O 1s at 531.1 and 529.6 eV, which corresponded to the adsorbed surface oxygen (Oads) and the lattice oxygen (Olatt), respectively.8 The Oads/Olatt ratio was 51.04/48.96. After adsorption, as shown in Fig. 8(b), two peaks were detected at the same position over the used LaMnO3/Ce0.7Sn0.3O2 sample. However, the ratio of Oads/Olatt decreased to 49.98/50.02. The surface oxygen was more active than the lattice oxygen based on previous studies.28 The oxygen took part in the Hg0 oxidation process, which is in agreement with the experiment results.
Fig. 8(c) displays the Mn 2p XPS spectra over the fresh LaMnO3/Ce0.7Sn0.3O2 sample, the double main peaks were ascribed to Mn 2p1/2 and Mn 2p3/2. Based on the analysis, the fitting peaks at 644.0 and 642.0 were ascribed to Mn4+ species and Mn3+ species, respectively.8 No peak could be fitted to Mn2+ by analysis calculation. The ratio of Mn4+/Mn3+ was 31.88/68.12. The previous studies indicated that the higher valance of Mn, the higher the catalytic oxidation for Hg0.28 MnO2 was considered the primary phase of Mn-based material for Hg0 oxidation. After adsorption, as the spectra shown in Fig. 8(d), it was interesting that, there was only one fitting peak at 642.3 eV, which corresponded to Mn3+. Obviously, the used material lost Mn4+ species due to the reduction of Mn4+ to Mn3+ (Mn4+ + e− → Mn3+).
In addition, the Hg 4f spectra of the used LaMnO3/Ce0.7Sn0.3O2 sample were shown in Fig. 8(e). Two character peaks at 100 to 110 eV can be ascribed to Hg–O on the surface of the material.29 This further indicated that the mercury removal process was primarily due to chemical adsorption.
The Hg-TPD curves were collected to present the property of mercury binding on the surface of LaMnO3-based material. As shown in Fig. 9, the Hg-TPD curves were collected under 5 °C min−1. The desorption peaks of LaMnO3/SnO2 and LaMnO3/CeO2 were centered at 371.4 and 342.3 °C, respectively. Without CeO2 or SnO2 as the catalyst support, the desorption peak of LaMnO3 was at 360.8 °C. However, it was obvious that the desorption peak of LaMnO3/Ce0.7Sn0.3O2 was quite different. There were two peaks at about 300.0 and 697.5 °C, suggesting that two forms of mercury combined with LaMnO3/Ce0.7Sn0.3O2. The higher desorption temperature indicated stronger interaction forces between the mercury and adsorption sites. The definite bonding styles of mercury can be calculated based on the first-principle theory, which will be performed in our further study. The Hg-TPD results also provided us regeneration method for mercury desorption.
 | ||
| Fig. 9 Hg-TPD curves of LaMnO3/SnO2, LaMnO3/CeO2, LaMnO3 and LaMnO3/Ce0.7Sn0.3O2 at the heating rate of 5 °C min−1. | ||
3.3 Hg0 removal mechanism over LaMnO3/Ce0.7Sn0.3O2
| Hg0(g) → Hg0(ads) | (2) |
Hg0(ads) + 2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn4+ + Mn4+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O2− → 2 O2− → 2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn3+ + Mn3+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Hg–O Hg–O
| (3) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) represented the adsorption state.
represented the adsorption state.As the active center of the catalyst, LaMnO3 showed high catalytic activity for Hg0 oxidation due to its special crystal structure.14 Lanthanum-based perovskite oxides had demonstrated remarkable catalysis performance due to the higher redox behavior, oxygen mobility and ionic conductivity.30 As illustrated in Fig. 10, oxygen vacancies (where “■” represented oxygen vacancies) can be introduced to the structure to facilitate oxygen transfer and thus increased oxygen mobility.31 O2 introduced to oxygen vacancies and formed O2−, which was favorable for Hg0 oxidation and adsorption. This mechanism also indicated that the Hg0 removal efficiency decreased when there were no O2 in the simulated gas.
With the addition of SnO2, the performance at high temperature was enhanced. SnO2 was believed to be an efficient catalyst that makes full use of O2 in the simulated gas at high temperatures (>200 °C).20,21 O2 in the simulated gas can be adsorbed and exist as physical-adsorption (p-ads) O2. SnO2 had super electron transfer performance, which could offer electrons for O2, the O2 (p-ads) can be formed O2− then to O− and O2− by getting electrons. The O2 (p-ads) changed to chemical-adsorption O2, which was beneficial for Hg0 capture. During this process, the electrons came from the reduction of Mn4+ to Mn3+. The adsorbed O2− on the surface of catalysts was binding site for the oxidized mercury. The mechanism for O2 transformation can be illustrated as follows:
| O2(g) → O2(p-ads) | (4) |
| O2(p-ads) + e− → O2−(c-ads) | (5) |
| O2−(c-ads) + e− → 2O−(c-ads) | (6) |
| O−(c-ads) + e− → O2−(c-ads) | (7) |
The abovementioned reactions occurred at the temperatures higher than 200 °C. Therefore, the performance of LaMnO3 at high temperatures (>200 °C) can be enhanced after SnO2 acted as catalyst support.
Moreover, CeO2 was believed to have high oxygen storage capacity. The adsorbed oxygen can be stored in CeO2. On the one hand, the sufficient oxygen was beneficial for mercury adsorption. On the other hand, SnO2 can adsorb O2 from simulated gas for CeO2 utilization. In addition, CeO2 can re-oxidize the reduced Mn (Mn3+) to Mn4+, and Ce2O3 can be re-oxidized to CeO2 by O2. So the catalyst can be re-used. The equation can be illustrated as follows:
2Mn3+ + 2CeO2 → 2Mn4+ + Ce2O3 + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O2− O2−
| (8) |
| Ce2O3 + 1/2O2(g) → 2CeO2 | (9) |
The interaction between Sn and Ce oxides is illustrated in Fig. 10. O2 in the simulated gas can be captured by Ce–Sn binary oxides when O2− is given to LaMnO3 for the capture of mercury. The reduced Ce (Ce3+) or Sn (Sn2+) can be re-oxidized by the adsorbed-O2. The catalyst support is regenerated. Furthermore, based on the H2-TPR results, Ce and Sn also enhanced the reducibility of LaMnO3. CeO2 and SnO2 benefited the regeneration of reduced Mn. The interaction between Sn, Ce and Mn can be illustrated as follows:
| Sn4+ + 2Ce3+ ↔ Sn2+ + 2Ce4+ | (10) |
| Sn2+ + Mn4+ ↔ Sn4+ + Mn3+ | (11) |
| Ce3+ + Mn4+ ↔ Ce4+ + Mn3+ | (12) |
4. Conclusions
Herein, Ce1−xSnxO2 binary metal oxides were synthesized and evaluated for the catalytic oxidation removal of Hg0. The results indicated that Ce0.7Sn0.3O2 had the best performance for Hg0 removal. LaMnO3/Ce0.7Sn0.3O2 presented the highest performance among the as-prepared materials. The surface area was enlarged, the oxygen vacancies were promoted and the reducibility was enhanced when Ce0.7Sn0.3O2 acted as the catalyst support. LaMnO3 were the main active sites for Hg0 removal, but it lost its activity when the temperature was higher than 200 °C. SnO2 enhanced the high-temperature performance because it can adsorb O2 in the simulated gas to generate active O2−. CeO2 enhanced the oxygen storage capacity and enhanced the catalyst performance. The Ce–Sn catalyst support-modified materials will be a potential catalyst not only for Hg0 removal, but also for other catalytic oxidation reactions.Acknowledgements
This study was supported by the Major State Basic Research Development Program of China (973 Program, No. 2013CB430005), the National Natural Science Foundation of China (No. 51478261 and No. 51278294). This work was also funded by Collaborative Innovation Center of Environmental Pollution Control and Ecological Restoration, Henan Province (XTCX-002). Thanks for Shanghai Tongji Gao Tingyao Environmental Science and Technology Development Foundation.References
- Z.-w. Wang, Z.-s. Chen, D. Ning and X.-s. Zhang, J. Environ. Sci., 2007, 19, 176–180 CrossRef CAS.
- J. H. Pavlish, E. A. Sondreal, M. D. Mann, E. S. Olson, K. C. Galbreath, D. L. Laudal and S. A. Benson, Fuel Process. Technol., 2003, 82, 89–165 CrossRef CAS.
- S. Wang, L. Zhang, G. Li, Y. Wu, J. Hao, N. Pirrone, F. Sprovieri and M. Ancora, Atmos. Chem. Phys., 2010, 10, 1183–1192 CrossRef CAS.
- A. L. Soerensen, R. P. Mason, P. H. Balcom, D. J. Jacob, Y. Zhang, J. Kuss and E. M. Sunderland, Environ. Sci. Technol., 2014, 48, 11312–11319 CrossRef CAS PubMed.
- L. Zhao, C. Li, X. Zhang, G. Zeng and J. Zhang, Catal. Sci. Technol., 2015, 5, 3459–3472 CAS.
- J. Li, N. Yan, Z. Qu, S. Qiao, S. Yang, Y. Guo, P. Liu and J. Jia, Environ. Sci. Technol., 2009, 44, 426–431 CrossRef PubMed.
- J. Xie, H. Xu, Z. Qu, W. Huang, W. Chen, Y. Ma, S. Zhao, P. Liu and N. Yan, J. Colloid Interface Sci., 2014, 428, 121–127 CrossRef CAS PubMed.
- H. Xu, Z. Qu, C. Zong, W. Huang, F. Quan and N. Yan, Environ. Sci. Technol., 2015, 49(11), 6823–6830 CrossRef CAS PubMed.
- L. Xing, Y. Xu and Q. Zhong, Energy Fuels, 2012, 26, 4903–4909 CrossRef CAS.
- H. Li, C.-Y. Wu, Y. Li and J. Zhang, Appl. Catal., B, 2012, 111, 381–388 CrossRef.
- D. Jampaiah, K. M. Tur, P. Venkataswamy, S. J. Ippolito, Y. M. Sabri, J. Tardio, S. K. Bhargava and B. M. Reddy, RSC Adv., 2015, 5, 30331–30341 RSC.
- D. Jampaiah, S. J. Ippolito, Y. M. Sabri, B. M. Reddy and S. K. Bhargava, Catal. Sci. Technol., 2015, 5, 2913–2924 CAS.
- S. Cimino, S. Colonna, S. De Rossi, M. Faticanti, L. Lisi, I. Pettiti and P. Porta, J. Catal., 2002, 205, 309–317 CrossRef CAS.
- S. Royer, D. Duprez, F. Can, X. Courtois, C. Batiot-Dupeyrat, S. Laassiri and H. Alamdari, Chem. Rev., 2014, 114, 10292–10368 CrossRef CAS PubMed.
- H. Najjar and H. Batis, Appl. Catal., A, 2010, 383, 192–201 CrossRef CAS.
- H. Xu, J. Xie, Y. Ma, Z. Qu, S. Zhao, W. Chen, W. Huang and N. Yan, Fuel, 2015, 140, 803–809 CrossRef CAS.
- H. Chang, X. Chen, J. Li, L. Ma, C. Wang, C. Liu, J. W. Schwank and J. Hao, Environ. Sci. Technol., 2013, 47, 5294–5301 CrossRef CAS PubMed.
- C. Liu, H. Xian, Z. Jiang, L. Wang, J. Zhang, L. Zheng, Y. Tan and X. Li, Appl. Catal., B, 2015, 176, 542–552 CrossRef.
- C. He, B. Shen, J. Chen and J. Cai, Environ. Sci. Technol., 2014, 48, 7891–7898 CrossRef CAS PubMed.
- S. Park, S. An, Y. Mun and C. Lee, ACS Appl. Mater. Interfaces, 2013, 5, 4285–4292 CAS.
- J. Yan, E. Khoo, A. Sumboja and P. S. Lee, ACS Nano, 2010, 4, 4247–4255 CrossRef CAS PubMed.
- Y. Gao, Z. Zhang, J. Wu, L. Duan, A. Umar, L. Sun, Z. Guo and Q. Wang, Environ. Sci. Technol., 2013, 47, 10813–10823 CrossRef CAS PubMed.
- T. Meng, M. Ara, L. Wang, R. Naik and K. S. Ng, J. Mater. Sci., 2014, 49, 4058–4066 CrossRef CAS.
- L. Abello, B. Bochu, A. Gaskov, S. Koudryavtseva, G. Lucazeau and M. Roumyantseva, J. Solid State Chem., 1998, 135, 78–85 CrossRef CAS.
- F. Buciuman, F. Patcas and T. Hahn, Chem. Eng. Process., 1999, 38, 563–569 CrossRef CAS.
- Z.-Y. Pu, J.-Q. Lu, M.-F. Luo and Y.-L. Xie, J. Phys. Chem. C, 2007, 111, 18695–18702 CAS.
- J. Nie and H. Liu, J. Catal., 2014, 316, 57–66 CrossRef CAS.
- H. Xu, Z. Qu, S. Zhao, J. Mei, F. Quan and N. Yan, J. Hazard. Mater., 2015, 299, 86–93 CrossRef CAS PubMed.
- S. Yang, Y. Guo, N. Yan, D. Wu, H. He, Z. Qu and J. Jia, Ind. Eng. Chem. Res., 2011, 50, 9650–9656 CrossRef CAS.
- H. Zhu, P. Zhang and S. Dai, ACS Catal., 2015, 5(11), 6370–6385 CrossRef CAS.
- J. T. Mefford, W. G. Hardin, S. Dai, K. P. Johnston and K. J. Stevenson, Nat. Mater., 2014, 13, 726–732 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The detailed information about materials characterization can be found. See DOI: 10.1039/c6ra10006f |
| This journal is © The Royal Society of Chemistry 2016 |