In situ encapsulation of Pd crystals inside foam-like carbon films continuously filled with α-Fe: investigating the nucleation of FePd3 alloys†
Filippo S. Boi*a,
Jian Guoa,
Mu Lana,
Gang Xianga,
Yi Heb,
Shanling Wangb and
Hongmei Chenc
aCollege of Physical Science and Technology, Sichuan University, Chengdu, China. E-mail: f.boi@scu.edu.cn
bAnalytical and Testing Centre, Sichuan University, Chengdu, China
cCollege of Chemistry and Materials Science, Sichuan Normal University, Chengdu, China
First published on 1st June 2016
Abstract
A key challenge in the fabrication and encapsulation of FePd alloys inside carbon materials is the achievement of continuous FexPdx filling rates. We propose an advanced approach which allows the encapsulation of Pd crystals into foam-like carbon films continuously filled with α-Fe and the subsequent nucleation of the FePd3 alloys. The alloying-mechanism is also investigated.
The synthesis of low anisotropy FexPdx alloys inside carbon nanotubes (CNTs) has recently opened new avenues toward the encapsulation of tetragonal FePd alloys for the achievement of giant coercivities.1,2 CNTs can indeed be considered ideal capsules which can guarantee a continuous protection of the encased particles from the external environment, easy particle-dispersion and manipulation.3–13 The encapsulation of these alloys can be achieved through chemical vapor deposition (CVD) of a Pd-containing hydrocarbon (dichloro-cyclooctadiene palladium) with ferrocene.1 However despite the advantage of a better dispersion inside the CNT-core, the encapsulation of these phases in the form of continuous nanowires is still a challenge due to (i) the alloying mechanism of Fe and Pd and (ii) the concentration of Cl radicals which can strongly affect the catalyst by etching interactions. Carbon diffusion has been generally described as the rate limiting step for the formation of CNTs nanostructures.14 Yokoyama, et al. reported carbon diffusion values three to six times faster in Pd than in γ-Fe, Ni and Co.15 Furthermore the terminal solid solubility of interstitial carbon in Pd was reported to be in the same order of magnitude as those in nickel and cobalt.15 However despite the high solubility, it has also been shown that the Pd catalyst is reluctant to form stable carbide phases. Also, the formation of FePd-type alloys appears to be kinetically more favorable than that of two separate carbide phases.1 In this letter we propose an alternative approach which allows the direct encapsulation of Pd crystals into carbon-based foam-like films continuously filled with α-Fe.16 In the method presented in this letter dichloro-cyclooctadiene palladium is evaporated at the temperature of 350 °C and pyrolyzed at the temperature of 900 °C on the top of the foam-like film. We demonstrate the successful deposition of Pd-containing amorphous-carbon-structures on the foam-surface by scanning electron microscopy (SEM), backscattered electrons (BE), energy dispersive X-rays (EDX) and X-ray diffraction (XRD). The obtained films were then annealed firstly at the temperatures of 1000 °C and 600–650 °C under an Ar/H2 environment at ambient pressure. The successful formation of the FePd3 phase in the form of large islands of 1–8 micrometres size is demonstrated through SEM, EDX, BE, XRD, transmission electron microscopy (TEM), scanning TEM (STEM) and STEM-EDX analyses. Also we show that further annealing experiments performed directly in situ through XRD measurements at the temperatures of 25 °C, 150 °C, 300 °C and 400 °C in vacuum values below 7 Pa did not show changes in the FePd3 phase. The magnetic properties of the annealed foam are demonstrated through temperature dependent VSM analyses (see ESI† for detailed experimental conditions and used instrumentation). The Pd deposition was achieved by evaporation (T = 350 °C) and pyrolysis (T = 900 °C) of 90 mg of dichloro-cyclooctadiene palladium on the top of foam-like films continuously filled α-Fe (previously prepared through CVD of ferrocene/dichlorobenzene at 650–770 °C (ref. 16)) inside a CVD reactor of length 1.5 m and inner diameter approximately 40 mm (ref. 16) under an Ar flow of 15 ml min−1. In Fig. 1A and B ESI† the SEM and BE confirm the morphological quality of the as grown foam-like films continuously filled with α-Fe (bright areas in Fig. 1B ESI†). The morphological variation of the foam like film after Pd-deposition through the CVD reaction with dichloro-cyclooctadiene palladium was then investigated. As shown in Fig. 1 the reaction successfully allowed the deposition of Pd-phases encapsulated in amorphous carbon (confirmed by TEM ESI Fig. 4†) on the surface of the carbon based foam.
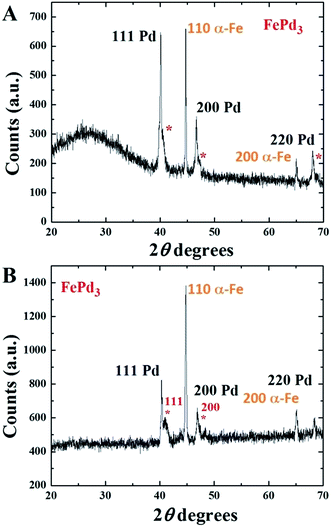
The area indicated by the red circle in Fig. 1A refers to the deposited-Pd phase while the area indicated by the pink circle refers to the foam-like film filled with α-Fe. This interpretation is confirmed by EDX of the two areas shown in Fig. 1B and C. These analyses revealed the following % of Fe and Pd phases: in Fig. 1B 99% of Fe and 1% of Pd; in Fig. 1C 86% of Pd and 14% of Fe (excluding for simplicity the contribution of carbon from the foam). The observation of a very high % of Pd in Fig. 1C confirms the presence of a large quantity of Pd catalyst on the surface of the foam-like film. Further confirmation of this interpretation was then sought by XRD measurements. As shown in Fig. 2A the XRD analyses clearly revealed the presence of the face centered cubic structure of Pd together with the body centered cubic structure of α-Fe (note that the band at low-angle refers to a partially amorphous arrangement of the Fe phase in the foam). Interestingly 3 more not-well defined peaks could be also found (see red stars). The position of these peaks can be associated to that of a FePd3 phase with the 111, 200 and 220 reflections respectively. In order to enhance the intensity of the FePd3 peaks the use of annealing methods was then considered.
The annealing of a fraction of the produced films was performed in Ar/H2 (g.p. 1 bar) at the temperatures of 1000 °C for 1 h and 650 °C for 6 h. The result of the annealing experiment was then analyzed through XRD as shown in Fig. 2B. Interestingly the XRD measurements revealed an increase in the intensity of the FePd3 111 and 200 peaks suggesting therefore that the used annealing conditions can allow a possible diffusion and subsequent chemisorption of the Pd particles into the α-Fe filling. This process can be explained considering that both the Fe and Pd phases are in a melted status at the temperature of 1000 °C and can therefore interact to form FexPdx alloys. This interpretation is also confirmed by the SEM, BE and EDX analyses performed on the as annealed sample, as shown in Fig. 3A–F.
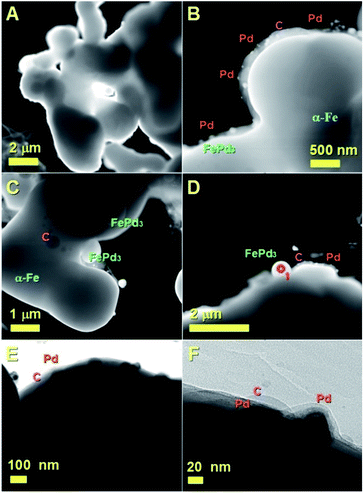
In Fig. 3A and B some of the areas of FePd3 nucleation are indicated with the red stars. Two examples can be found in Fig. 3C and E with the elemental composition in Fig. 3D and F. The % of Fe and Pd was 74% and 26% in the case of Fig. 3D and 54% and 46% in the case of Fig. 3E. Further EDX analyses were also performed in other areas of the foam as shown in ESI Fig. 2,† where in (A) an example of Pd-catalyst island chemisorbed in the Fe-filling of the foam is shown with BE. In this case the % of Pd rescaled with respect to that of Fe (excluding for simplicity the contribution of carbon from the foam and oxygen from impurities) can be found to be 95% (5% of Fe). Instead in the case of (B) an alloyed area is analyzed. In this case the EDX analyses revealed the values of 62% of Fe and 38% of Pd. Note that due to contributions of the nearest Fe-catalyst areas, the EDX results can not be used to accurately estimate the stoichiometry and structure of the encapsulated alloy, which can be instead understood by the XRD structural analyses shown above. These interpretations were also confirmed by the STEM and TEM measurements. In Fig. 4A the cross-sectional analyses confirm the continuous filling rate of the foam-like film.
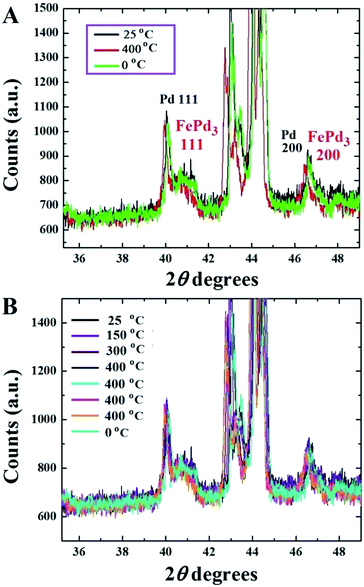
In Fig. 4B the observation of Pd particles into the amorphous carbon layers of the foam clearly confirm that these particles can migrate into the film-filling at high temperatures through diffusion and subsequently form the FePd3 alloy by interacting with the α-Fe phase. This interpretation was also confirmed by the different contrast of the areas in the foam (see Fig. 4C and D) and by EDX as shown in ESI Fig. 3.† The STEM observations were further confirmed by the bright field TEM images in Fig. 4E and F which revealed the morphology of the Pd particles encapsulated in the carbon foam-like film with an increasing level of detail proving therefore (i) the diffusion of the Pd particles into the foam and (ii) the subsequent alloy-formation. These analyses clearly confirm that this method can be very useful for future studies on phase-manipulation of FePd alloys. Further evaluation of the foam structure was then considered through Raman spectroscopy. The majority of these analyses revealed the absence of graphitic ordering (see Fig. 5 ESI†) in agreement with the XRD observations (Fig. 2B) where no graphitic peak was found. However in few areas of the foam an intense D band and a very weak G band (weak graphitic ordering) could be observed (see Fig. 6 ESI†). In order to further understand the mechanism of alloy formation, more annealing experiments were considered through temperature dependent XRD at the temperatures of 25 °C, 150 °C, 300 °C, 400 °C. Due to the instrument limitations the maximum temperature that could be investigated was 400 °C. The use of a fast cooling method was also considered to create possible distortions in the structure of the FePd3 alloy by cooling to 0 °C in 5–10 min. However as shown in Fig. 5, in this case no changes were found in the structure of the foam-phases.
Note that the unlabeled peaks correspond to the signal of the substrate (the α-Fe 110 peak is covered by the substrate peaks in the same scanning angle-range). We believe that the absence of changes in the FePd3 phase can be associated to (1) the lower temperature of annealing and (2) to the much lower value of the pressure inside the annealing chamber (below 7 Pa). The analysis of the magnetic properties of the annealed foam-like film was then considered at 50 K, 150 K and 300 K. As shown in ESI Fig. 7† a low value of coercivity of approximately 55 Oe which decreases to 47 Oe at room temperature is found. Furthermore values of saturation magnetization of 200 emu g−1 at 50 K and 193 emu g−1 at 300 K (lower with respect to those reported in the case of the only α-Fe16) were found due to the presence of the FePd3 phase. These analyses suggest therefore that the produced foam-like film is an ideal candidate for the fabrication and future manipulations of continuous FePd-type alloys. Future studies will focus in finding the conditions required for the transformations of the FePd3 alloy into the L10 type alloys.
Acknowledgements
We acknowledge Prof. Gong Min for his continuous support and the National Natural Science Foundation of China Grant No. 11404227.References
- F. S. Boi, J. Guo, M. Lan, G. Xiang, S. Wang, J. Wen and S. Zhang, Carbon, 2015, 95, 634 CrossRef CAS.
- N. T. T. Van, T. T. Trung, N. H. Nam, N. D. Phu, N. H. Ha and N. H. Luong, Eur. Phys. J.: Appl. Phys., 2013, 64, 10403 CrossRef.
- U. Weissker, S. Hampel, A. Leonhardt and B. Buchner, Materials, 2010, 3, 4387 CrossRef CAS.
- S. Hampel, A. Leonhardt, D. Selbmann, K. Biedermann, D. Elefant, Ch. Muller, T. Gemming and B. Buchner, Carbon, 2006, 44, 2316 CrossRef CAS.
- H. Terrones, F. López-Urías, E. Muñoz-Sandoval, J. A. Rodríguez-Manzo, A. Zamudio and A. L. Elías, et al., Solid State Sci., 2006, 8, 303 CrossRef CAS.
- A. Leonhardt, M. Ritschel, R. Kozhuharova, A. Graff, T. Muhl, R. Huhle, I. Monch, D. Elefant and C. M. Schneider, Diamond Relat. Mater., 2003, 12, 790 CrossRef CAS.
- N. Grobert, M. Mayne, M. Terrones, J. Sloan, R. E. Dunin-Borkowski, R. Kamalakaran, T. Seeger, H. Terrones, M. Ruhle, D. R. M. Walton, H. W. Kroto and J. L. Hutchison, Chem. Commun., 2001, 471–472 RSC.
- F. C. Dillon, A. Bajpai, A. Koos, S. Downes, Z. Aslam and N. Grobert, Carbon, 2012, 50, 3674 CrossRef CAS.
- A. Leonhardt, M. Ritschel, M. Elefant, D. N. Mattern, K. Bie-dermann, S. Hampel, Ch. Muller, T. Gemming and B. Buchner, J. Appl. Phys., 2005, 98, 074315 CrossRef.
- R. Lv, S. Tsuge, X. Gui, K. Takai, F. Kang, T. Enoki, J. Wei, J. Gu, K. Wang and D. Wu, Carbon, 2009, 47, 1141 CrossRef CAS.
- W. Wang, K. Wang, R. Lv, W. J. Zhang, X. Kang, F. Chang, J. Chang, Q. Shu, Y. Wang and D. Wu, Carbon, 2007, 45, 1105–1136 CrossRef.
- R. Lv, F. Kang, J. Gu, X. Gui, J. Wei, K. Wang and D. Wu, Appl. Phys. Lett., 2008, 93, 223105 CrossRef.
- X. Gui, K. Wang, W. Wang, J. Wei, X. Zhang, R. Lv, Y. Jia, Q. Shu, F. Kang and D. Wu, Mater. Chem. Phys., 2009, 113, 634–637 CrossRef CAS.
- R. T. K. Baker and P. S. Harris, Chemistry and physics of carbon, Marcel Dekker, New York, 1978 Search PubMed.
- H. Yokoyama, H. Numakura and M. Koiwa, Acta Mater., 1998, 46, 2823 CrossRef CAS.
- F. S. Boi, J. Guo, M. Lan, T. Yu, S. Wang, Y. He, J. Wen and G. Xiang, Carbon, 2016, 101, 28 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra09983a |
| This journal is © The Royal Society of Chemistry 2016 |