Deposition of silver nanoparticles onto two dimensional BiOCl nanodiscs for enhanced visible light photocatalytic and biocidal activities†‡
Abstract
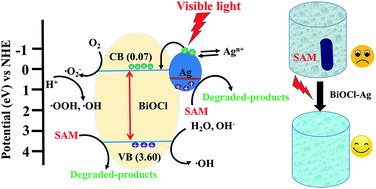
Two dimensional (2D) BiOCl nanodiscs were synthesized as a photocatalyst by a one-step solvothermal method. To enhance its photocatalytic activity, Ag nanoparticles (Ag NPs) were deposited on the surface of BiOCl to form BiOCl–Ag heterostructured nanocomposites. Synthesized samples were characterized by X-ray diffraction (XRD), Raman spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), photoluminescence (PL) spectroscopy and UV-vis absorption spectroscopy. Sulfanilamide (SAM) was chosen as a model chemical to evaluate the photocatalytic performance of both materials under visible irradiation. Results show that the photo-degradation efficiency of SAM was improved to 82.4% using BiOCl–Ag as compared to pristine BiOCl, indicating Ag deposition is an effective way to augment the photocatalytic activity of BiOCl. As an excellent antibacterial agent, Ag, on the other hand, may enhance the biocidal capability of BiOCl. Taking E. coli and B. subtilis as target bacteria, though BiOCl displayed certain antibacterial function under visible light irradiation (2 log reduction), BiOCl–Ag was demonstrated to have a significantly higher efficiency with 100% bactericidal rate. This study shows that Ag modified BiOCl nanodiscs can be a promising material in the application of disinfection as well as degradation of sulfonamide derived antibiotics.

 Please wait while we load your content...
Please wait while we load your content...