Synthesis and characterization of a novel 99mTc analogue of *I-mIBG showing affinity for nor-epinephrine transport positive tumors
Abstract
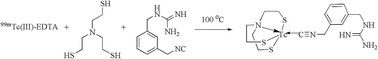
Radio-iodine (123I/131I) labeled meta-iodobenzylguanidine (mIBG) is a proven radiopharmaceutical used for diagnosis of neuroendocrine tumors (NET) related to neural crest origin. Here, a 99mTc analogue of *I-mIBG using 99mTc-4+1 labeling approach has been synthesized and evaluated for its potential in NET imaging. This involved preparation of benzylguanidine precursor (mono-dentate donor) which was isolated in a five step synthetic procedure. The precursor was designed such that, in presence of a tetra-dentate NS3 co-ligand, [99mTcO4]− and Sn2+ reducing agent, it formed the desired 99mTc-4+1 complex. Complex formation was identified by radio-HPLC and structural details were affirmed by characterizing its rhenium analogue. Bio-evaluation studies of the complex were carried, both in vitro and in vivo, and the results obtained were compared with no-carrier added-125I-mIBG (nca-125I-mIBG). In vitro studies, in SK-N-SH neuroblastoma cell line showed affinity of the tracer towards nor-epinephrine transporters. Although the absolute uptake was lower compared to nca-125I-mIBG, its specificity was similar (∼90%) as the uptake reduced on inhibition with specific transport blocker, desmethylimipramine (DMI). Biodistribution studies in normal Wistar rats, pre-treated with mIBG and DMI, respectively, showed reduced myocardial uptake than its control. The results thus obtained merits high potential of synthesized 99mTc-4+1 complex for NET imaging.


 Please wait while we load your content...
Please wait while we load your content...