Antifouling PVDF membrane grafted with zwitterionic poly(lysine methacrylamide) brushes
Dapeng Liu,
Jing Zhu,
Ming Qiu and
Chunju He*
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai, 201620, P. R. China. E-mail: chunjuhe@dhu.edu.cn; Fax: +86-21-67792855; Tel: +86-21-67792842
First published on 15th June 2016
Abstract
Antifouling PVDF membranes were fabricated through the covalent binding of lysine methacrylamide (LysAA) brushes on the membrane surface via mussel-inspired surface-initiated atom transfer radical polymerization (SI-ATRP). The zwitterionic pLysAA brushes were immobilized on the membrane surface as well as the inner pore surface, which was conducive to enhance the hydrophilicity and separation properties of PVDF membranes due to the strong hydration capacity of zwitterions and amide groups in the brushes. Upon modification, the wetting and antifouling properties of the graft membranes were improved significantly with the water contact angle reduced to as low as 37° and the water flux recovery ratio increased to as high as 95%. This work provides an effective alternative to the traditional poly(ethylene glycol) or poly(betaine)-based materials for the fabrication of low-fouling membranes, which may find its application in blood purification, protein separation and water treatment.
1. Introduction
During the past few decades, membrane technology has become one of the most popular separation technologies due to its simplicity in operation, low energy consumption, no phase changes or chemical additives, etc.1 However, all existing synthetic membrane materials that possess ideal physicochemical stability are hydrophobic, and contaminants are easily absorbed on the membrane surface. Thus membrane fouling has become a common hindrance to membrane technology, which can raise operating costs, diminish process productivity and shorten membrane life.2 Therefore, to develop membranes that can resist fouling is of great importance.A great deal of polymer materials, e.g. polyacrylamide,3,4 poly(2-hydroxyethyl methacrylate),5–8 polysaccharide,9 poly(acrylic acid)10 etc., have been explored for the preparation of antifouling membranes via coating, grafting or blending methods. Among these materials, poly(ethylene glycol) (PEG) and its derivative polymers are the most widely studied materials due to their hydrophilicity, biocompatibility and easy controlled characters.11–14 However, the instability of PEG toward oxidation, especially in the presence of oxygen and transition metal ions, limits its further application.15–17
In the past few years, substantial efforts have been devoted to the development of zwitterionic surfaces to reduce or eliminate nonspecific adsorption, mimicking the zwitterionic character of phospholipid cell membranes. Zwitterionic poly(sulfobetaine), poly(carboxybetaine) and poly(phosphobetaine) have been extensively studied for the modification of hydrophobic membranes18–21 due to their ultralow fouling properties since those polymers can bind a significant number of water molecules through electrostatic and hydrogen bonding interactions, resulting in the formation of a strong surface hydration layer to effectively exclude proteins from the surface.22
Recently, amino acid-based antifouling materials have attracted much attention due to their zwitterionic and biomimetic nature as well as ultralow biofouling properties.23–26 But only a few works have focused on amino acid-based materials for the antifouling modification of membranes. Hadidi et al. grafted lysine on cellulose membrane, which showed a very low degree of protein fouling.27 Shi grafted lysine, serine and glycine molecules on flat sheet PAN-based ultrafiltration membrane via carbodiimide chemistry, while only the membrane grafted with lysine maintaining its zwitterionic structure showed superior antifouling capacities.28 Glycine-functionalized PVA was also grafted on PES ultrafiltration membranes, which showed enhanced flux recovery ratio.29 However, little attention has been devoted to zwitterionic polymer brushes based on amino acids for the antifouling modification of hydrophobic membranes.
In this study, zwitterionic polymer based on lysine i.e. poly(lysine methacrylamide) (pLysAA) was employed for the fabrication of antifouling PVDF membranes. Zwitterionic brush-like pLysAA was grafted onto the surface of PVDF membranes via dopamine-initiated atom transfer radical polymerization, which was known as an effective and convenient technique for the synthesis of uniform polymer brushes with controlled characteristics. ATR-FTIR and XPS were used to confirm the presence of the zwitterionic brushes on membrane surface. Cyclic filtration and protein adsorption experiments were carried out to test the antifouling properties. The effects of zwitterionic pLysAA brushes on membrane morphology, wettability, permeability and selectivity were also investigated in detail.
2. Materials and methods
2.1 Materials
L-Lysine hydrochloride (98%), methacryloyl chloride (97%), 8-hydroxyquinoline (98%), cupric carbonate basic, 2-bromoisobutyryl bromide (BIBB, 98%), 3-hydroxytyramine hydrochloride and 2,2′-bipyridyl (Bpy, 99.5%) were purchased from Energy Chemical (Shanghai, China). Bovine serum albumin (BSA), N,N-dimethylacetamide (DMAc) and tris(hydroxymethyl)aminomethane (Tris) were obtained from Sinopharm Chemical Reagent (Shanghai, China). Copper(I) bromide (CuBr, 98%) was purchased from Aldrich and purified according to a standard procedure.30 Poly(vinylidene fluoride) powder supplied by Arkema Inc. was dried at 100 °C for 24 h. Triethylamine (TEA) was evaporated before use. Lysine methacrylamide (LysAA) was synthesized according to a procedure reported previously.31,32 All other chemicals were used as received.2.2 Preparation of PVDF-g-PLysAA membranes
The pure PVDF ultrafiltration membranes were prepared with non-solvent induced phase separation method from a DMAc solution containing 17 wt% PVDF powder with poly(ethylene glycol) (PEG 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, PVDF/PEG weight ratio = 5
000, PVDF/PEG weight ratio = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the pore-forming agent. The prepared PVDF membranes were firstly coated with polydopamine using a simple dip-coating procedure. Briefly, dopamine (2.0 g L−1) was dissolved in 10 mM Tris–HCl solution (pH 8.5). PVDF membranes were immersed into the dopamine solution and shaken at 30 °C for about 4 h. Then the membranes were taken out and washed thoroughly with deionized water and anhydrous ethanol alternatively before dried at 40 °C in vacuum oven for 24 h.
2) as the pore-forming agent. The prepared PVDF membranes were firstly coated with polydopamine using a simple dip-coating procedure. Briefly, dopamine (2.0 g L−1) was dissolved in 10 mM Tris–HCl solution (pH 8.5). PVDF membranes were immersed into the dopamine solution and shaken at 30 °C for about 4 h. Then the membranes were taken out and washed thoroughly with deionized water and anhydrous ethanol alternatively before dried at 40 °C in vacuum oven for 24 h.
The polydopamine-coated PVDF membrane was placed into a three-neck round bottomed flask with triethylamine (1.60 g, 15.8 mmol) and dichloromethane (100 mL). Then, 2-bromoisobutyryl bromide (3.45 g, 15 mmol) was added dropwise to the stirred solution at 4 °C. After stirred at room temperature for 12 h, the membrane was taken out from the solution and washed successively with dichloromethane, ethanol and water.
Finally, a series of PVDF-g-PLysAA membranes were synthesized by surface initiated atom transfer radical polymerization (SI-ATRP). For example, the initiator-immobilized membrane of about 17.0 cm2 in surface area was placed into a 50.0 mL aqueous solution with 3.0 g of LysAA monomer before a purified argon stream was introduced to degas the solution. Then Bpy (0.063 g, 0.4 mmol) and CuBr (0.028 g, 0.2 mmol) were added into the flask under argon atmosphere. After three freeze–pump–thaw cycles, the polymerization was carried out at 30 °C under constant stirring for a predetermined period of time. The resultant membranes were rinsed extensively with water and methanol, and stored in deionized water before use. The grafting yield (mg cm−2) was determined by the weight change of the membrane per area. The resultant membranes were marked by M-1, M-3 and M-5 corresponding to the various reaction times (h). For comparison, the pure, polydopamine-coated and BIBB-immobilized membranes were marked by M-0, M-D and M-Br respectively.
2.3 Surface characterization
The chemical composition of PVDF-g-PLysAA membranes was characterized using an attenuated total reflectance-Fourier transform infrared spectrometer (ATR-FTIR, Nicolet 8700, America) with zinc selenide (ZnSe) as an internal reflection element. The elemental composition of the membrane surface was characterized by X-ray photoelectron spectroscopy (XPS) performed on a Thermal Scientific K-Alpha spectrometer with a monochromated Al K X-ray source (1486.6 eV photons). Surface morphologies of the pure and modified membranes were observed using scanning electron microscopy (SEM, HITACHI S-3000, Japan) and atomic force microscopy (AFM, BioScope Catalyst, USA). The samples were freeze-dried for 24 h, fractured in liquid nitrogen for the cross-section, fixed on a sample holder and sputter-coated with gold before SEM observation. Static water contact angles (WCA) of the membranes were measured following the sessile drop method with a telescopic goniometer (Dataphysics OCA40, Germany) at 25 °C.2.4 Protein adsorption
FITC-labeled BSA (BSA-FITC) was prepared according to a reported procedure.33,34 The membranes (0.5 × 0.5 cm2) were incubated with 0.1 g L−1 FITC-BSA in PBS buffer at pH 7.4 and shaken in a dark place at 4 °C for 12 h. After that, the membranes were rinsed with PBS buffer solution thoroughly to remove the non-firmly adsorbed proteins and dried with a stream of nitrogen gas. The adsorption of BSA-FITC on the membranes was observed with a laser scanning confocal microscope (Lerca TCS SP5 II, Germany). All membranes were exposed to an excitation source of argon laser at 488 nm while the emission was collected in the range of 493–628 nm.2.5 Permeation experiments
The permeation properties of the membranes were characterized using a dead-end filtration system connected with a peristaltic pump and a solution reservoir. All of the membranes were initially pressured at 0.15 MPa with deionized water for at least 30 min. The volume of the permeated water was collected at 0.1 MPa. The stable flux (J) and BSA rejection (R) were calculated by the following equations:
 | (1) |
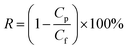 | (2) |
2.6 Antifouling assessment
To evaluate antifouling properties of the prepared membranes, a three-cycle filtration experiment was conducted with BSA as the model pollutant. The permeate was collected at an interval of 5 min at 0.1 MPa. Firstly, the membrane was pressured at 0.15 MPa before water flux was recorded within 30 min. Then, the feed was replaced with 1.0 g L−1 BSA in PBS solution (pH 7.4) and BSA solution flux was recorded within 30 min. After that, the tested membrane was washed with deionized water for 5 minutes before another filtration cycle. Flux recovery ratio (Frr) of the sample was calculated by the following equation:
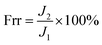 | (3) |
3. Results and discussion
It is well known that dihydroxyphenylalanine (DOPA), one of the mussel adhesive proteins, plays a dominant role in imparting adhesive characteristics. As one of the derivatives of DOPA, dopamine can also form a dense coating on various substrates via self-polymerization, which makes it one of the most promising strategies for membrane fabrication and modification.35 There are plenty of catechol groups on polydopamine-coated membrane surface and inner pore surface, which can be used for the immobilization of alkyl halide ATRP initiator and subsequently SI-ATRP of hydrophilic monomers.3.1 Surface grafting
As shown in Fig. 1, the pure PVDF membranes were firstly coated with polydopamine, and the resultant hydroxyl groups on the membrane surface and inner pore surface were used for the immobilization of alkyl halide ATRP initiator. Antifouling membranes, PVDF-g-PLysAA, were prepared through surface-initiated atom transfer radical polymerization of lysine-based zwitterionic monomer i.e. lysine methacrylamide (LysAA).Grafting yield of the pLysAA brushes on membrane surface is one of the key factors that affect the antifouling properties of the graft membranes. With SI-ATRP, grafting yield of pLysAA can be easily controlled by varying the grafting time. As shown in Fig. 2, grafting yield on membrane surface increases monotonically with time, indicating that the brush growth polymerization is living and well-controlled. After 12 h of polymerization, the grafting yield can reach as high as 0.25 mg cm−2, which is in a similar range to that reported by others.17,36 By varying the grafting time, properties of the graft membranes can be easily controlled.
3.2 Surface compositions of the membranes
The surface chemical compositions of the pure and modified membranes were characterized by attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectroscopic measurement and the spectra were presented in Fig. 3. The presence of the grafted pLysAA could be ascertained from the amide groups observed from the bands of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (amide I) at 1621 cm−1 and N–H bending (amide II) at 1530 cm−1.37 The bands at 3340 cm−1 and 2935 cm−1 can be assigned to N–H and C–H stretching vibrations respectively.31 Moreover, the relative intensity of the above characteristic peaks obviously increases with the grafting yield. These results indicate that pLysAA brushes have been successfully grafted onto membrane surface.
O stretching (amide I) at 1621 cm−1 and N–H bending (amide II) at 1530 cm−1.37 The bands at 3340 cm−1 and 2935 cm−1 can be assigned to N–H and C–H stretching vibrations respectively.31 Moreover, the relative intensity of the above characteristic peaks obviously increases with the grafting yield. These results indicate that pLysAA brushes have been successfully grafted onto membrane surface.
To further confirm the polydopamine coating and pLysAA immobilization, X-ray photoelectron spectroscopy (XPS) was employed to monitor the variation of the surface chemistry. As shown in Fig. 4 and Table 1, for the pure PVDF membrane, two major peaks at 688.1 and 286.7 eV are ascribed to the binding energy of F 1s and C 1s respectively. For the polydopamine-coated membrane (M-D), a new peak appears at 400.4 eV for N 1s, indicating successful coating of polydopamine on membrane surface. For M-Br, a new peak at 70.4 eV for Br 3d is obvious, indicating the successful immobilization of 2-bromoisobutyryl bromide on membrane surface. The oxygen-to-nitrogen signal ratio (O/N = 2.81) of M-D is a little higher than that of the theoretical value of dopamine (O/N = 2.00) due to the presence of oxygen in the substrate membrane. After the grafting polymerization, the oxygen-to-nitrogen signal ratio is decreased to 1.80, which is between the theoretical values of dopamine and the monomer LysAA (O/N = 1.00), resulting from the immobilization of LysAA on the surface of the graft membranes.
| Sample | Element (at%) | |||||
|---|---|---|---|---|---|---|
| C | F | O | N | Br | O/N ratio | |
| M-0 | 64.39 | 33.62 | 1.99 | — | — | — |
| M-D | 67.58 | 18.00 | 10.64 | 3.79 | — | 2.81 |
| M-Br | 68.80 | 14.87 | 11.96 | 3.81 | 0.56 | 3.14 |
| M-5 | 73.94 | 0.98 | 16.12 | 8.96 | — | 1.80 |
The XPS C 1s and N 1s core-level spectra of M-5 are shown in Fig. 5. For the C 1s spectrum, there are mainly five components via curve fitting. The peaks with binding energies of 284.6 eV (C–H/C–C) and 290.2 eV (C–F) are associated with the chemical structure of PVDF. The peak with binding energy of 288.4 eV (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N–C
O/N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) is associated with the chemical structure of the grafted pLysAA brushes.25 The N 1s core-level spectrum recorded for the graft membrane can be fitted using two components at binding energies of 399.7 eV and 401.5 eV, corresponding to C–NH2/O
O) is associated with the chemical structure of the grafted pLysAA brushes.25 The N 1s core-level spectrum recorded for the graft membrane can be fitted using two components at binding energies of 399.7 eV and 401.5 eV, corresponding to C–NH2/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N and C–NH3+ species.38
C–N and C–NH3+ species.38
3.3 Surface morphology
The morphologies of the pure and modified membranes were observed by SEM, as shown in Fig. 6. For the polydopamine-coated membrane i.e. M-D, pores are depressed slightly compared with the pure membrane. After the graft polymerization, pore size of the graft membranes declines further with the increasing grafting time, owning to the coverage of the grafted chains. For the cross-sections of the pure and modified membranes, typical asymmetrical structures consisting of a top layer and a porous sub-layer can be observed. No obvious changes can be observed in the macrovoids of the membranes (see Fig. 6b) while varying pore architecture can be seen in the amplified pore walls (see Fig. 6c). It is indicated that the graft polymerization can also take place in the sub-layers. | ||
| Fig. 6 SEM images of surface morphology (a), cross-sectional (b) and amplified morphology (c) of the pure and modified membranes. | ||
The typical three-dimensional AFM surface topography images of the pure and modified membranes were also studied and presented in Fig. 7. Mean RMS of the pure membrane is about 49.8 nm. After coating of polydopamine, the surface becomes denser and the RMS value correspondingly decreases to 25.2 nm. After grafting polymerization, the RMS of the graft membranes can further decrease to 21.2 nm. These results indicate that the surface of the modified membranes becomes slightly smoother than that of the original one.
3.4 Membrane hydrophilicity
Membrane hydrophilicity plays an important role on the antifouling properties of membranes, since a hydrophilic surface tends to resist the adsorption of pollutants. Fig. 8 represents the water contact angle (WCA) of pure and modified membranes and its change as a function of drop age. The initial WCA of pure PVDF membrane is as high as 93.1°, which is decreased to about 84.4° after dopamine modification. After grafting polymerization, the initial WCA of the membranes is decreased significantly to as low as 37°. All of the WCA decreases with drop age, but it is notable that the WCA of the graft membranes decreases much faster than that of the pure PVDF membrane. After 3 min, the WCA of the graft membranes can reach as low as 25.0°.These results indicate that the introduction of pLysAA brushes brings enhanced hydrophilicity to hydrophobic PVDF membrane. It should be noted that the enhanced hydrophilicity of the graft membranes are mainly ascribed to the excellent hydration capacity of zwitterion and amide groups in the brushes instead of surface roughness, since membrane surface becomes smoother after grafting polymerization while the zwitterion and amide groups can bind a significant number of water molecules through electrostatic and hydrogen bonding interactions.
3.5 Protein adsorption
Protein adsorption or convective deposition on membrane surface is usually in accordance with the antifouling performance of membranes.39 Thus deposition of FITC-labeled BSA on membrane surface was investigated. As shown in Fig. 9, lots of fluorescence aggregations can be observed in the image of M-0, indicating significant BSA-FITC adsorption on membrane surface. Compared with the pure membrane, fluorescence aggregations on the graft membranes are decreased significantly. This is in association with the reduced extent of protein adsorption on the membrane surface. The grafted pLysAA brushes on membrane surface can bind lots of water molecules resulting in the formation a protective hydration layer on membrane surface, which protects the membranes from BSA.3.6 Filtration experiments
To better understand the effects of grafted pLysAA brushes on membrane properties, filtration experiments were conducted. As shown in Fig. 10, water flux of the polydopamine-coated membrane is higher than that of the pure membrane due to the enhanced hydrophilicity while those of the graft membranes are decreased consecutively after grafting polymerization, which is in accordance with the characteristics of grafting modification. Generally, water flux is mainly affected by surface hydrophilicity and membrane pore structure. Pore narrowing has become the dominant factor for the membranes after grafting since the grafting polymerization occurs not only on the membrane surface but also in membrane pore channels, leading to the value of water flux decreases with grafting time. However, the separation efficiency of the graft membranes increases rapidly with the increasing of grafting time. BSA rejection ratio is increased from 44% for the pure membrane to as high as 94% for the graft membranes.3.7 Antifouling assessment
In order to simulate the fouling situation during filtration, a three-cycle-filtration experiment was conducted with BSA as the model pollutant. As shown in Fig. 11, all of the membranes show irreversible flux decline after the filtration of BSA solution. However, there is almost no decline for the water flux of M-5 after 3 cycles of filtration. Flux recovery ratio of the membranes is increased remarkably to as high as 95% after the introduction of zwitterionic pLysAA brushes, as shown in Fig. 12. These results are in accordance with the hydrophilicity and protein adsorption performances of the pure and modified membranes discussed above. The incorporated lysine-based zwitterionic brushes on membrane surface as well as pore channel surface preferentially adsorb water molecules due to the strong hydration capabilities of zwitterions and hydrophilic amide groups. As a result, a dense hydration layer is proposed to be formed on the surface of the graft membranes, which protects the membranes from pollutants.4. Conclusions
In this work, zwitterionic pLysAA brushes were covalently immobilized on the surface and pore channels of hydrophobic PVDF membranes via mussel-inspired surface-initiated atom transfer radical polymerization, which is conducive to enhance the antifouling properties of the membranes. With the optimal grafting yield, the introduction of zwitterionic pLysAA brushes can significantly improve the hydrophilicity of the membranes and strongly suppress BSA adsorption. Accordingly the antifouling properties of the graft membranes are enhanced remarkably with flux recovery ratio as high as 95%. These results indicate that pLysAA can be considered to be an effective alternative to the traditional poly(ethylene glycol) and poly(betaine)-based materials for the antifouling modification of hydrophobic membranes.Acknowledgements
This work is supported by grants from the National Science Foundation of China (No. 21174027), Program for New Century Excellent Talents in University (No. NCET-12-0827) and Program of Introducing Talents of Discipline to Universities (No. 111-2-04).References
- G. D. Kang and Y. M. Cao, J. Membr. Sci., 2014, 463, 145–165 CrossRef CAS.
- S. Liang, G. Qi, K. Xiao, J. Sun, E. P. Giannelis, X. Huang and M. Elimelech, J. Membr. Sci., 2014, 463, 94–101 CrossRef CAS.
- W. Li, J. Zhou, J. S. Gu and H. Y. Yu, J. Appl. Polym. Sci., 2010, 115, 2302–2309 CrossRef CAS.
- Z. Xue, S. Wang, L. Lin, L. Chen, M. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270–4273 CrossRef CAS PubMed.
- Y. Sui, X. Gao, Z. Wang and C. Gao, J. Membr. Sci., 2012, 394–395, 107–119 CrossRef CAS.
- J. Ju, C. Wang, T. Wang and Q. Wang, J. Colloid Interface Sci., 2014, 434, 175–180 CrossRef CAS PubMed.
- W. Yandi, S. Mieszkin, P. Martin-Tanchereau, M. E. Callow, J. A. Callow, L. Tyson, B. Liedberg and T. Ederth, ACS Appl. Mater. Interfaces, 2014, 6, 11448–11458 Search PubMed.
- D. Keskin, J. I. Clodt, J. Hahn, V. Abetz and V. Filiz, Langmuir, 2014, 30, 8907–8914 CrossRef CAS PubMed.
- T. Mohan, R. Kargl, K. E. Tradt, M. R. Kulterer, M. Braćić, S. Hribernik, K. Stana-Kleinschek and V. Ribitsch, Carbohydr. Polym., 2015, 116, 149–158 CrossRef CAS PubMed.
- W. Zhang, Y. Zhu, X. Liu, D. Wang, J. Li, L. Jiang and J. Jin, Angew. Chem., Int. Ed., 2014, 53, 856–860 CrossRef CAS PubMed.
- A. Venault, Y. H. Liu, J. R. Wu, H. S. Yang, Y. Chang, J. Y. Lai and P. Aimar, J. Membr. Sci., 2014, 450, 340–350 CrossRef CAS.
- A. Bera, R. M. Gol, S. Chatterjee and S. K. Jewrajka, Desalination, 2015, 360, 108–117 CrossRef CAS.
- T. Yuan, J. Meng, T. Hao, Y. Zhang and M. Xu, J. Membr. Sci., 2014, 470, 112–124 CrossRef CAS.
- Y. F. Zhao, L. P. Zhu, Z. Yi, B. K. Zhu and Y. Y. Xu, J. Membr. Sci., 2014, 470, 148–158 CrossRef CAS.
- J. Cui, Y. Ju, K. Liang, H. Ejima, S. Lorcher, K. T. Gause, J. J. Richardson and F. Caruso, Soft Matter, 2014, 10, 2656–2663 RSC.
- E. Ostuni, R. G. Chapman, R. E. Holmlin, S. Takayama and G. M. Whitesides, Langmuir, 2001, 17, 5605–5620 CrossRef CAS.
- Y. C. Chiang, Y. Chang, A. Higuchi, W. Y. Chen and R. C. Ruaan, J. Membr. Sci., 2009, 339, 151–159 CrossRef CAS.
- T. Xiang, R. Wang, W. F. Zhao, S. D. Sun and C. S. Zhao, Langmuir, 2014, 30, 5115–5125 CrossRef CAS PubMed.
- R. Zhou, P. F. Ren, H. C. Yang and Z. K. Xu, J. Membr. Sci., 2014, 466, 18–25 CrossRef CAS.
- H. Meng, Q. Cheng and C. Li, Appl. Surf. Sci., 2014, 303, 399–405 CrossRef CAS.
- Q. Zhou, X. P. Lei, J. H. Li, B. F. Yan and Q. Q. Zhang, Desalination, 2014, 337, 6–15 CrossRef CAS.
- J. B. Schlenoff, Langmuir, 2014, 30, 9625–9636 CrossRef CAS PubMed.
- A. M. Alswieleh, N. Cheng, I. Canton, B. Ustbas, X. Xue, V. Ladmiral, S. Xia, R. E. Ducker, O. El Zubir, M. L. Cartron, C. N. Hunter, G. J. Leggett and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 9404–9413 CrossRef CAS PubMed.
- Q. Liu, A. Singh and L. Liu, Biomacromolecules, 2013, 14, 226–231 CrossRef CAS PubMed.
- P. Lin, L. Ding, C.-W. Lin and F. Gu, Langmuir, 2014, 30, 6497–6507 CrossRef CAS PubMed.
- W. Li, Q. Liu and L. Liu, Langmuir, 2014, 30, 12619–12626 CrossRef CAS PubMed.
- M. Hadidi and A. L. Zydney, J. Membr. Sci., 2014, 452, 97–103 CrossRef CAS.
- Q. Shi, Y. Su, W. Chen, J. Peng, L. Nie, L. Zhang and Z. Jiang, J. Membr. Sci., 2011, 366, 398–404 CrossRef CAS.
- F. Li, J. Ye, L. Yang, C. Deng, Q. Tian and B. Yang, Appl. Surf. Sci., 2015, 345, 301–309 CrossRef CAS.
- R. N. Keller, H. D. Wycoff and L. E. Marchi, Inorg. Synth., 1946, 2, 1–4 Search PubMed.
- S. Nagaoka, A. Shundo, T. Satoh, K. Nagira, R. Kishi, K. Ueno, K. Iio and H. Ihara, Synth. Commun., 2005, 35, 2529–2534 CrossRef CAS.
- Q. Liu, W. Li, A. Singh, G. Cheng and L. Liu, Acta Biomater., 2014, 10, 2956–2964 CrossRef CAS PubMed.
- A. S. Blawas, T. F. Oliver, M. C. Pirrung and W. M. Reichert, Langmuir, 1998, 14, 4243–4250 CrossRef CAS.
- J. B. Delehanty, K. M. Shaffer and B. Lin, Anal. Chem., 2004, 76, 7323–7328 CrossRef CAS PubMed.
- H. C. Yang, J. Luo, Y. Lv, P. Shen and Z. K. Xu, J. Membr. Sci., 2015, 483, 42–59 CrossRef CAS.
- Y. Chang, W. J. Chang, Y. J. Shih, T. C. Wei and G. H. Hsiue, ACS Appl. Mater. Interfaces, 2011, 3, 1228–1237 Search PubMed.
- G. J. G. Davies, D. P. Knight and F. Vollrath, PLoS One, 2013, 8, 1–7 Search PubMed.
- O. Cavalleri, G. Gonella, S. Terreni, M. Vignolo, L. Floreano, A. Morgante, M. Canepa and R. Rolandi, Phys. Chem. Chem. Phys., 2004, 6, 4042–4046 RSC.
- T. Wang, Y. Q. Wang, Y. L. Su and Z. Y. Jiang, J. Membr. Sci., 2006, 280, 343–350 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |











