DOI:
10.1039/C6RA08901A
(Paper)
RSC Adv., 2016,
6, 61423-61433
Removal of crystal violet from aqueous solutions using functionalized cellulose microfibers: a beneficial use of cellulosic healthcare waste
Received
7th April 2016
, Accepted 19th June 2016
First published on 22nd June 2016
Abstract
In this research, the preparation of functionalized cellulosic microfibers (FCMFs) was proposed as a beneficial use of cellulosic healthcare waste for the removal of crystal violet from aqueous solutions. The functionalization process was performed using a sulfonation reaction by chlorosulfonic acid. The results of scanning electron microscopy revealed a significant deformation in the fibrous structure of cellulose as a result of the sulfonation process. The functionalization of cellulosic fibers was confirmed by investigation of the FTIR spectra of cotton and FCMFs. The charge density of FCMFs was calculated from their sulfur content which was measured using a CHNS elemental analyzer. The influence of factors affecting the adsorption process such as pH, salt content, contact time, initial dye concentration and temperature were examined. The adsorption capacity was increased by increasing the salt content and temperature. Equilibrium was achieved after 60 min of mixing. The equilibrium adsorption data were well described by Freundlich, Redlich–Peterson isotherms. The maximum adsorption capacity was found to be 872 mg g−1. The adsorption kinetic data were well fitted to pseudo-second-order and Elovich kinetic models. Thermodynamic parameters indicated that the adsorption process was spontaneous and endothermic in nature. The residual root-mean-square error (RMSE), average absolute relative error (AARE), cross-correlation coefficient (CCC, R) and chi-square test (χ2) were used to evaluate the fitting of the adsorption isotherms and kinetic models to the experimental results. The saturated adsorbent was regenerated by acid washing (HCl, 1 mol L−1) and removal efficiency was decreased slightly in each batch adsorption/regeneration cycle.
Introduction
Large amounts of synthetic dyes are produced annually over the world.1 Dyes are generally classified as anionic, cationic and non-ionic. Cationic dyes are more toxic than anionic ones2 and most of them are toxic and have carcinogenic and mutagenic effects on human beings3 and can lead to irreversible damage to aquatic life.4 Among the cationic dyes, crystal violet (CV) is a well-known dye which is widely used in the textile dyeing and printing industries. It has also been widely used as a disinfectant and fungicide in aquaculture due to its low cost and high effectiveness.5 CV is toxic to human cells and is classified as a mutagenic substance.6
A variety of synthetic dyes have been observed in the effluents of some industries such as coloring, textile and tanning. The improper discharge of wastewater from the related industries to the environment can lead to a reduction in sunlight penetration, photosynthetic activity, dissolved oxygen concentration and water quality, and subsequently serious damage to aquatic life.7 Therefore, finding efficient methods to remove dyes from waters and wastewaters is of critical importance for sustainable development. In recent years, various treatment technologies have been developed for dye removal from industrial wastewaters which are classified as chemical (ozonation,8 chemical oxidation,9 electrochemical,10 photocatalytic degradation11), physical (adsorption,12 coagulation/flocculation,13 ion exchange,14 membrane filtration15) and biological16 treatments. Biological techniques are not efficient in dye removal due to toxic effects of dyes on the microorganism activities. Chemical treatment possesses drawbacks such as formation of by-products, which may be more toxic than the original substance. Adsorption process is one of the most efficient and successful methods that have been successfully employed for dye removal from wastewaters due to its simplicity, the facile scaling-up, ease of operation, high efficiency without releasing any by-product to the environment and the possibility of recovering the adsorbent as well as availability of a wide range of the adsorbents.17,18 The only disadvantage of adsorption processes is sludge production which this problem can be alleviated or can be overcome completely through usage of high capacity adsorbents or regeneration of saturated adsorbents.
Several nanomaterial-based adsorbents have been recently developed for dye removal from wastewaters, including carbon nanotube,19 graphene oxide20 and functionalized nanoparticles.21 These adsorbents have high adsorption capacity as a result of small particle size and subsequently high surface area. However, there is great concern regarding adverse effects of nanoparticles on the environment and human binges which limits their applications in water and wastewater treatment.22,23 Activated carbon is the most widely used adsorbent for dye removal. However, commercially available activated carbon is very expensive and has high regeneration cost. The natural materials and agricultural/industrial waste materials have been extensively noticed because of importance of sustainable development.24
Among the natural materials, biopolymers have been recently investigated for preparation of biodegradable adsorbents, Biodegradable polymer-based adsorbents, including starch-graft-poly(acrylic acid) hydrogel,25 chitosan magnetic composite microspheres,26 glutamic acid modified chitosan magnetic composite microspheres,26 carboxylate-functionalized cellulose nanocrystals,27 alginate/acid activated bentonite composite beads,28 guar gum grafted sodium acrylate,29 kappa-Carrageenan beads30 have been developed for removal of crystal violet from aqueous samples.
Cellulose is one of the most abundant and significant fibrous material in nature which has high mechanical strength, high surface area, biodegradability, non-toxicity and low cost.31 Cellulosic materials including bandages, gauze and cotton are single-use disposable items, which are extensively used in hospitals and medical clinics. The healthcare wastes (HCWs) are not recycled due to health considerations. Therefore, proper treatment and disposal is important to protect the environment and public health. Incineration, disinfection, sterilization, plasma arc and land filling have been adopted for treatment of HCWs in different parts of the world. In most of the developing countries, infectious and pathological wastes are incinerated rarely with required air pollution control which can lead to release of large amount of greenhouse gases to the environment.32 Disposal of the HCWs by burial in landfill may cause the pollution of groundwater. Preparation of an adsorbent for wastewater treatment from cellulosic healthcare wastes (CHCWs) seems to be an appropriate and attractive alternative to conventional disposal methods. Despite the high surface area of cellulose, its adsorption capacity towards the cationic dyes is low due to lack of suitable functional groups.
In this work, FCMFs was prepared from CHCWs through sulfonation reaction and its adsorptive performance was studied in batch experiments. The effect of factors affecting the absorption process such as pH, salt content, contact time, adsorbent dosage and temperature were examined. The equilibrium, kinetics and thermodynamics of the adsorption process were also studied. The residual root-mean-square error (RMSE), average absolute relative error (AARE), cross-correlation coefficient (CCC, R) and chi-square test (χ2) were used to evaluate the fitting of the adsorption isotherms and kinetic models to the experimental results. Regeneration of adsorbent was investigated in batch mode. Spectrophotometric detection was used to determine the dye concentration.
Materials and methods
Materials
CV (C.I. 42555, linear formula: C25H30N3Cl, molecular weight: 407.98 g mol−1 and λ max: 590 nm) obtained from Sigma-Aldrich (St. Louis, MO) was of analytical reagent grade. Stock solution of CV with concentration of 1000 mg L−1 was prepared by dissolving the appropriate amount of related salt in deionized water and was kept in dark. The pH adjustment was performed using NaOH and HCl purchased from Merck (Darmstadt, Germany). Chlorosulfonic acid (CSA, 97% wt) and Dimethylformamide (DMF, 99% wt) were supplied by Merck (Darmstadt, Germany) and were used for functionalization of cellulosic microfibers. All obtained reagents, except CV were of extra pure grade and were used without further purification. Medical cotton waste was obtained from health center of University of Tehran and was dried at 323 K for 1 h. Deionized water was used throughout the whole experiments.
Preparation of FCMFs
Cellulose microfibers were functionalized by sulfonation of dried medical cotton waste in DMF medium. CSA was used as a sulfonation reagent. The effect of CSA/Cotton ratio and the reaction time were investigated and optimized. In a typical procedure, 12 g of cotton waste was immersed in 150 mL of DMF in a dry and clean beaker for 15 min at ambient temperature. Then, 33 g of CSA was slowly added to 150 mL of DMF in an ice bath. This procedure was followed by slowly adding this mixture to the cotton immersed in DMF. Final mixture in a sealed glass flask was shaken at a constant speed of 120 rpm at ambient temperature. Sampling was performed after 1, 2 and 3 h of shaking. After filtration over a 1 μm membrane, the remaining solid on the filter was washed several times with deionized water, and then it was transferred to a flask and neutralized by adequate amount of sodium bicarbonate solution. After that, it was filtered and washed several times with deionized water. In order to obtain the optimum mass ratio of CSA/cotton, the sulfonation reaction was performed at different mass ratios (1.5, 3.0 and 4.0) for 2 h. The FCMFs is highly hydrophilic and as a consequence of contact with water, it comes in gel form.
Schematic process for sulfonation of cellulose and the photographs of cotton and FCMFs are shown in Fig. 1. It should be noted that in the reaction medium, all microorganisms and viruses which may present in the CHCWs are destroyed.
 |
| | Fig. 1 Sulfonation of cellulose (a), photographs of (b) cotton and (c) FCMFs. | |
Characterization of adsorbent
Scanning electron microscopy (SEM) was used for microstructure analysis of FCMFs (SEM, model: HITACHI S-4160). The functionalization of cellulosic fibers was confirmed by investigation of FTIR spectra of cotton and FCMFs. The charge density of FCMFs was calculated from its sulfur content which was measured by CHNS elemental analyzer (Eager 300, EA 1112).
Batch adsorption experiments
In order to investigate the effect of pH on the adsorption efficiency, 0.02 g of adsorbent was added to 100 mL of a dye solution at concentration level of 300 mg L−1, and the pH of medium were adjusted to different values (2–12) by adding dilute HCl or NaOH solutions. The mixture was agitated on a stirrer at 150 rpm at 298 K for 60 min. For studying the effect of other important factors, the adsorption experiments were also performed at various levels of factors (salt concentration; 0–5% w/v, initial dye concentration; 50–350 mg L−1, contact time; 0–60 min, and temperature; 283–333 K). To investigate the adsorption kinetics, 100 mL-portions of a 200 mg L−1 dye solution containing of 0.02 g of adsorbent were mixed at pH 7, and stirred for various time intervals at 298 K. For investigating the adsorption isotherms, 100 mL-portions of dye solutions with different concentrations (150–450 mg L−1) containing of 0.02 g of adsorbent were mixed at pH 7, and stirred for 1 h at 298 K. The dye concentration was determined at the maximum wavelength of 590 nm using a UV/VIS spectrophotometer (HACH, DR 5000, USA). The removal efficiency and the amount of CV adsorbed per unit weight of adsorbent at the equilibrium (qe, mg g−1) were calculated using eqn (1) and (2), respectively.| |
 | (1) |
| |
 | (2) |
where Co and Ce (mg L−1) are initial and equilibrium concentration of CV, respectively, V (L) is the volume of solution, and m (g) is the mass of adsorbent.
Statistical analysis
In addition to correlation coefficient (R2), the residual root-mean-square error (RMSE), average absolute relative error (AARE), cross-correlation coefficient (CCC, R) and the chi-square test (χ2), were used to evaluate the fitting of the adsorption isotherms and kinetic models to the experimental results. The related equations are as follow:| |
 | (3) |
| |
 | (4) |
| |
 | (5) |
| |
 | (6) |
where qe,exp is the experimental results, qe,cal is the calculated values according to the model, n is the number of experimental data, and p is the number of parameters within model equation. RMSE and AARE should be as small as possible and R should be close to unity for better predictability. When more than one model is acceptable statistically then the chi-square (χ2) test is required to be performed to find the best-fit model. The lowest value indicates the best model33
Results and discussion
Characterization of FCMFs
The electrostatic interaction between cationic dyes and anionic sites of adsorbent is the main driving force for adsorption of cationic dyes. The adsorption capacity increases by increasing the charge density of adsorbent. The negative charge of adsorbent is as a result of sulfate functional groups which were incorporated on the surface of cellulose fibers through sulfonation reaction. The charge density of FCMFs was indirectly determined from its sulfur content measured by CHNS elemental analyzer. The effect of reaction time and CSA/Cotton ratio on the charge density and adsorption capacity are shown in Fig. 2. As can be seen, the charge density and adsorption capacity were increased by increasing the reaction time and CSA/cotton ratio. According to the results presented in Fig. 2, reaction time of 2 h and CSA/Cotton ratio of 3 were chosen as the optimum condition for preparation of FCMFs with a high adsorption capacity. The charge density of prepared FCMFs at the optimum condition was 2310 μeq. g−1 which is equivalent to 861 mg g−1.
 |
| | Fig. 2 (a) Effect of reaction time on the capacity and charge density of FCMFs; the mass ratio of CSA/cotton was kept constant at 3 (b) effect of CSA/cotton ratios on the capacity and charge density; reaction time was kept at 2 h. | |
In addition to adsorption capacity, biodegradability of cellulose sulphate can be affected by substitution degree or functional group density. Cellulose sulfate is a well-known biodegradable polymer34,35 and it would be degraded by the enzymes. The higher substitution degree causes the stronger steric hindrance for the approach of cellulase to cellulose sulfate.36
The FCMFs is highly hydrophilic and as a consequence of contact with water, it comes in gel form. The higher charge density, the higher hydrophilicity and subsequently the higher gel strength. As can be seen in Fig. 3, by increasing the reaction time, the gel strength of FCMFs increased as a result of increase in charge density.
 |
| | Fig. 3 Effect of reaction time on the gel strength of FCMFs (a) 0.5 h, (b) 1 h, (c) 1.5 h and (d) 2 h. CSA/cotton: 2. | |
The microstructure of cellulose fibers and FCMFs were studied by scanning electron microscopy (SEM) and the obtained photos were presented in Fig. 4. A significant deformation in fibrous structure of cellulose was observed which is due to sulfonation process and hydrolysis in acidic medium.
 |
| | Fig. 4 SEM images of (a) cotton fibers and (b) FCMFs. | |
FTIR spectra of cotton and FCMFs are shown in Fig. 5. Sulfonation of cellulose was confirmed by the appearance of new IR bands at 997 and 1230 cm−1 which was respectively assigned to the symmetric and asymmetric stretching vibrations of sulfate group.
 |
| | Fig. 5 FTIR spectra of (a) cotton and (b) FCMFs. | |
Investigation of factors affecting the adsorption process
Effect of pH. The pH is regarded as a crucial parameter in the adsorption process since it may control the uptake mechanism of the adsorbate and can affect the adsorption sites on the surface of adsorbent.37 The effect of solution pH on the adsorption capacity of FCMFs was shown in Fig. 6. The adsorption capacity was independent of pH in the range of 2–8. In the alkaline medium, the removal efficiency was high because the concentration of OH− is high enough to lead to precipitation of CV. It is clear form Fig. 6 that H+ ions cannot compete with dyes cations for active sites of adsorbent due to high affinity of CV toward anionic functional groups of FCMFs. In addition to this, the sulfate functional group is classified as a neutral functional group; therefore it is anionic in the wide range of pH. As a consequence, the variation of pH has no effect on the charge density of sulfate-functionalized adsorbents, indicating insignificant interaction between H+ and active sites of adsorbent. While the charge density of adsorbents with weak acidic functional groups such as carboxylic group depends on the pH which decreases significantly by decreasing the pH.38 The subsequent experiments were performed in the neutral pH.
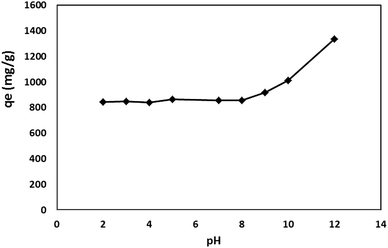 |
| | Fig. 6 Effect of pH on the adsorption capacity of FCMFs. Adsorbent: 200 mg L−1, dye concentration: 300 mg L−1, temperature: 298 K, contact time: 60 min. | |
Effect of salt content. Textile industrial effluents often contain electrolytes.39 The effect of electrolyte on the adsorption of CV by FCMFs was studied by measuring the adsorbed dye from the solution containing sodium chloride in the range of 0–5% (w/w). The amount of dye adsorbed was plotted versus salt concentration (Fig. 7). On increasing the concentration of the electrolyte up to 2% (w/w); adsorption performance was found to increase and then decreased slightly due to slight competitive effect between CV ions and other cations for adsorption by the active sites on the adsorbent. Therefore, the adsorbent cannot be regenerated through washing with a saline solution.
 |
| | Fig. 7 Effect of salt concentration on the adsorption capacity. Adsorbent: 200 mg L−1, pH: 7, dye concentration: 300 mg L−1, temperature: 298 K, contact time: 60 min. | |
Effect of contact time. The contact time between adsorbent and pollutant play a significant role in removal efficiency of pollutants from water and wastewater. A high adsorption rate indicates the high efficiency of the adsorbent for application in wastewater treatment. Batch adsorption experiments were carried out by stirring the dye solution containing adsorbent for a period ranging from 2–60 min. The results are shown in Fig. 8. Adsorption rate was high in the initial stages and became slow in later stages till saturation was attained. This is obvious from the fact that a large number of active sites are available for adsorption at the initial stages and after a lapse of time, the remaining active sites are difficult to be occupied because of repulsion between the solute molecules of the solid and bulk phases.39 The equilibrium was achieved after 50 min of mixing.
 |
| | Fig. 8 Effect of contact time on the adsorption capacity. Adsorbent: 200 mg L−1, dye concentration: 200 mg L−1, pH: 7, temperature: 298 K. | |
Effect of initial dye concentration. In order to understand the influence of initial dye concentration on the removal of CV by FCMFs, the batch experiments were performed at different initial concentrations in the range of 50–350 mg L−1. It is clear from Fig. 9 that the removal efficiency significantly decreased with increase in initial dye concentration, because the number of CV ions is higher than that of the active sites on the adsorbent. But at lower initial dye concentrations, there are sufficient active sites to adsorb CV completely. On the other hand, as the initial dye concentrations increased from 50 to 350 mg L−1, the adsorption capacity increased from 252 to 856 mg g−1. The initial dye concentration provides a powerful driving force to overcome the mass transfer resistance between the aqueous and solid phases.40
 |
| | Fig. 9 Effect of initial dye concentration on the removal efficiency and adsorption capacity. Adsorbent: 200 mg L−1, pH: 7, temperature: 298 K, contact time: 60 min. | |
Equilibrium studies
Adsorption isotherms are used to investigate the relationship between the amount of a substance adsorbed and its concentration in the solution after achieving equilibrium at constant temperature. The parameters obtained from different models provide important information on the adsorption mechanisms and the surface properties and affinities of the adsorbent.41 Freundlich and Langmuir models are the most commonly used adsorption equilibrium models; however there are numerous isotherm models available, including Dubinin–Radushkevich, Redlich–Peterson, Radke–Prausnitz and Temkin. In this research, the equilibrium data were analyzed with the mentioned models. The linear forms of investigated models and the obtained results are presented in Table 1. The performance of different models was evaluated by different error functions and the results are shown in Table 2.
Table 1 Isotherm parameters obtained with linear regression and error values for adsorption of CV onto FCMFs at 298 Ka
| Isotherm |
Equation |
|
Parameters |
| qm: maximum adsorption capacity reflected a complete monolayer (mg g−1), KL: Langmuir constant or adsorption equilibrium constant (L mg−1), 1/n: isotherm constant indicates the empirical parameter, KF: isotherm constant indicates the capacity parameter (mg g−1) (L mg−1)1/n, KR: (L g−1), αR (L mg−1) and g: Redlich–Peterson constants, qs: adsorption capacity (mg g−1), β: constant of Dubinin–Radushkevich (mol2 J−2), E: related to free energy (kJ mol−1), KRP: Radke–Prausnitz constant (L mg−1), R: 8.314 gas constant (J mol−1 K−1), T: absolute temperature (K). |
| Langmuir |
 |
(7) |
qm: 872 mg L−1 |
 |
KL: 0.34 L mg−1 |
| Freundlich |
 |
(8) |
1/nF: 0.034 |
| KF: 714 (mg g−1) (L mg−1)1/n |
| Temkin |
qe = B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A + B1 A + B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce |
(9) |
B1: 27.36 mg g−1 |
| A: 1.86 × 1011 L mg−1 |
| Dubinin–Radushkevich |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qs − βε2 qs − βε2 |
(10) |
qs: 837 mg g−1 |
 |
(11) |
β: 1.4 × 10−7 mol2 J−2 |
 |
(12) |
E: 1.89 kJ mol−1 |
| Redlich–Peterson |
 |
(13) |
g: 0.9667 |
| KR: 8000 L g−1 |
| αR: (111.6 L mg−1)g |
| Radke–Prausnitz |
 |
(14) |
m: 0.9659 |
| KRP: 1401 L mg−1 |
| qm: 713.8 mg g−1 |
Table 2 Performance of different isotherm s for adsorption of CV onto FCMFs at 298 K (qe,exp: 856 mg g−1)
| Isotherms |
R2 |
Error functions |
| CCC |
RMSE |
AARE |
χ2 |
| Langmuir |
0.9993 |
0.8457 |
167.17 |
0.0868 |
377 |
| Freundlich |
0.9086 |
0.9507 |
16.54 |
0.0144 |
1.65 |
| Redlich–Peterson |
0.9999 |
0.9489 |
16.82 |
0.0145 |
1.71 |
| Dubinin–Radushkevich |
0.6601 |
0.8013 |
32.39 |
0.0258 |
6.27 |
| Temkin |
0.8965 |
0.9469 |
17.14 |
0.0148 |
1.77 |
| Radke–Prausnitz |
0.9999 |
0.9505 |
16.56 |
0.0144 |
1.65 |
The Langmuir model assumes monolayer adsorption onto identical active sites of adsorbent with uniform adsorption energies and without lateral interaction and steric hindrance between the adsorbed molecules.42 Therefore, the Langmuir model can be used to estimate the maximum adsorption capacity corresponding to complete monolayer coverage on the adsorbent surface. The Freundlich isotherm assumes that the adsorption on surface is non-ideal and reversible, which is not restricted to the monolayer adsorption.37 Temkin considers the effects of some indirect adsorbate/adsorbate interactions on adsorption isotherms and suggests that because of these interactions, the heat of adsorption of all the molecules in the layer decreases linearly with coverage.43 The Dubinin–Radushkevich isotherm does not assume a homogeneous surface or constant adsorption potential. In contrast to Langmuir, it is not based on ideal assumptions such as equipotent of the sorption sites, absence of steric hindrance between adsorbed and incoming particles and surface homogeneity on microscopic level.44 Redlich–Peterson and Radke–Prausnitz models45 can be applied either in homogeneous or heterogeneous systems. These models incorporate three parameters into an empirical equation.46 A trial and error procedure has to be used to find the best fit to experimental data using maximization the coefficient of determination (R2) and minimization of the sum of squared errors. According to Table 2, if R2-values were only be used to determine the goodness of fit for the models, Langmuir, Redlich–Peterson and Radke–Prausnitz models were found to fit the data well. Regarding the CCC, RMSE, AARE and χ2 values, Freundlich, Redlich–Peterson, Temkin and Radke–Prausnitz models exhibited the lower errors than the others. The fitting degree followed the sequence: Freundlich = Radke–Prausnitz (best fit) > Redlich–Peterson > Dubinin–Radushkevich > Langmuir. The obtained maximum capacities using Langmuir, Dubinin–Radushkevich and Radke–Prausnitz models were 872, 837 and 714 mg g−1, respectively. The maximum capacity of Radke–Prausnitz is not in agreement with the experimental data. The E-value calculated using Dubinin–Radushkevich model was 1.89 kJ mol−1 (<8 kJ mol−1),47 which suggests that the CV physically adsorbed onto FCMFs through electrostatic interaction. But this value is not valid due to low fitness of corresponding model.
The essential characteristic of the Langmuir equation can be expressed in terms of dimensionless factor (RL). The value of RL indicates the nature of adsorption as unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1) or irreversible (RL = 0). The values of RL ranged from 0.006 to 0.019 for different initial concentrations of dye solution, indicating that the adsorption of CV on FCMFs was irreversible. The high ratio of α/β in the Elovich equation also indicates the irreversibility nature of adsorption.
The greater Langmuir constant, the higher affinity of adsorbate to adsorb on the surface of adsorbent. The relationship between qe and Ce is presented in Fig. 10(a). As can be seen, the low equilibrium concentrations correspond to high adsorbent capacities (qe), indicating the high affinity of CV towards the active sites of adsorbent. Therefore, the prepared adsorbent can significantly decrease the dye concentration (Fig. 10(b)).
 |
| | Fig. 10 The relationship between qe and Ce (a). The high affinity of CV towards the active sites of adsorbent; adsorbent: 300 mg L−1, pH: 7, dye concentration: 200 mg L−1, temperature: 298 K, contact time: 60 min (b). | |
Kinetic studies
The investigation of adsorption rate is essential to design an appropriate adsorption system. Namely the pseudo-first-order, the pseudo-second order, intraparticle diffusion, liquid film diffusion and Elovich models were tested. The linear forms of investigated models and the obtained results are presented in Table 3. The reliability of these kinetic models was determined by measuring the coefficients of determination (R2), residual root-mean-square error (RMSE), average absolute relative error (AARE), cross-correlation coefficient (CCC, R) and chi-square test (χ2) and the results are shown in Table 4. The experimental and corresponding calculated data using kinetic models are shown in Fig. 11. It is clear that the liquid film diffusion model exhibited poor fit to experimental data, whereas the corresponding CCC-value presented in Table 4 is very close to unity. As a consequence, CCC error function was not considered for subsequent evaluation of models. Pseudo-second-order and Elovich models exhibited good fit to experimental data and the fitting degree followed the sequence: pseudo-second-order and Elovich (best fitting) > intraparticle diffusion > liquid film diffusion > pseudo-first-order. Furthermore, the calculated value of qe using pseudo-second-order model (876 mg g−1) is close to the related experimental value (856 mg g−1).
Table 3 Adsorption kinetic parameters calculated by various kinetic models for adsorption of CV onto FCMFs at 298 K (qe,exp 856 mg g−1)a
| Model |
Equation |
|
Parameters |
| k1: rate constant of pseudo-first order adsorption (min−1), k2: second-order rate constant of adsorption (g mg−1 min−1), qe(calc): equilibrium capacity (mg g−1), kdif: rate constant of intraparticle diffusion (mg g−1 min−1/2), C: intercept of intraparticle diffusion, kfd: liquid film diffusion rate coefficients (min−1), α: (mg g−1 min−1) and β: (g mg−1) are the initial adsorption rate of the Elovich equation and the desorption constant related to the extent of surface coverage and activation energy constant for chemisorption. |
| Pseudo-first-order |
 |
(15) |
k1: 0.0783 min−1 |
| qe(calc): 306 mg g−1 |
| Pseudo-second-order |
 |
(16) |
k2: 5.24 × 10−4 g mg−1 min−1 |
| qe(calc): 876 mg g−1 |
| Intraparticle diffusion |
qt = kdift1/2 + C |
(17) |
kdif: 45.2 mg g−1 min−1/2 |
| C: 545 |
| Liquid film diffusion |
ln(1 − F) = −kfdt |
(18) |
kfd: 0.0783 min−1 |
| Elovich |
 |
(19) |
β: 0.0108 mg g−1 min−1 |
| α: 1.88 × 104 g mg−1 |
Table 4 Performance of different kinetic model for adsorption of CV onto FCMFs at 298 K (qe,exp 856 mg g−1)
| Kinetic models |
R2 |
Errors |
| CCC |
RMSE |
AARE |
χ2 |
| Pseudo-first-order |
0.8719 |
0.9768 |
666.4 |
0.8279 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 865 865 |
| Pseudo-second-order |
0.9995 |
0.9774 |
13.25 |
0.0113 |
2.56 |
| Intraparticle diffusion |
0.8804 |
0.9383 |
42.58 |
0.0522 |
18.12 |
| Liquid film diffusion |
0.8719 |
0.9888 |
241.6 |
0.2747 |
1447 |
| Elovich |
0.9779 |
0.9889 |
16.01 |
0.0165 |
3.17 |
 |
| | Fig. 11 The kinetics for adsorption of CV onto FCMFs. Adsorbent: 200 mg L−1, dye concentration: 200 mg L−1, pH: 7, temperature: 298 K. | |
The liquid–solid adsorption involves diffusion which mainly controls the rate of adsorption. Adsorption occurs through three sequential steps:48,49 (a) diffusion across the liquid film surrounding the adsorbent particles (external diffusion or film diffusion); (b) diffusion within the pores of adsorbent (internal diffusion or intra-particle diffusion); (c) adsorption of adsorbate at internal active sites. A plot of ln(1 − (qt/qe)) versus t should be a straight-line, if the film diffusion is the rate determining step in adsorption process. On the other hand, the rate is controlled by intraparticle diffusion when a plot of qt against t1/2 is a straight-line. The plot goes through the origin if the intraparticle diffusion is the only rate-limiting step.50 Otherwise, the adsorption kinetics may be controlled by film diffusion and intraparticle diffusion simultaneously. According to the obtained results, external diffusion has no effect on the rate of adsorption process. However, diffusion in the liquid contained in the pores of adsorbent can significantly affect the adsorption process. Cellulose sulfate is highly hydrophilic and as a consequence of contact with water, it comes in gel form. For gel-type adsorbents, the mass transfer is controlled by diffusion in the liquid film surrounding the adsorbent particles and/or diffusion within the pores of adsorbent. At low initial concentrations, film diffusion is likely to be controlling step. At high initial concentrations, intraparticle diffusion is more likely to control the mass transfer.51 The C-value in intraparticle diffusion model is related to the boundary layer thickness, i.e., the larger C-value, the greater is the boundary layer effect.52
The parameters of α and β in the Elovich equation are the initial adsorption rate and the desorption constant, respectively. As can be seen in Table 3, the initial adsorption rate is significantly higher than the desorption rate.
Thermodynamic studies
The thermodynamic parameters which were considered to characterize the adsorption process are the standard enthalpy (ΔHo, J mol−1), standard free energy (ΔGo, J mol−1) and standard entropy (ΔSo, J mol−1 K−1). The values of ΔGo, ΔHo and ΔSo can be obtained from the following equation:| |
 | (20) |
| |
ΔGo = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(Ke) ln(Ke)
| (21) |
| |
 | (22) |
where R is the universal gas constant (8.314 J mol−1 K−1), Ke is the thermodynamic equilibrium constant of the adsorption and T is the absolute temperature (K). The calculated values of ΔHo, ΔSo and ΔGo are given in Table 5. The positive value of ΔHo indicates endothermic nature of the adsorption process. The positive value of ΔSo shows increasing in randomness at solid/solution interface during sorption. The negative value of ΔGo indicates the spontaneous nature and feasibility of the adsorption process.
Table 5 Thermodynamic parameters for adsorption of CV onto FCS at pH 7 and initial dye concentration of 200 mg L−1
| T (K) |
Ke |
ΔGo (kJ mol−1) |
ΔHo (kJ mol−1) |
ΔSo (J mol−1 K−1) |
| 283 |
1.72 |
−4.04 |
7.12 |
39.64 |
| 293 |
1.84 |
−4.49 |
|
|
| 313 |
2.11 |
−5.50 |
|
|
| 333 |
2.15 |
−5.94 |
|
|
Desorption study
An efficient adsorbent should possess both high adsorption capacities as well as excellent desorption characteristics, to render the adsorbent economically viable. Desorption experiments were performed through shaking the saturated adsorbent in HCl solution (1 mol L−1) for 30 min. The treated adsorbent was washed with deionized water to nearly neutral and dried at 313 K for next use. As can be seen from Fig. 12, adsorption efficiency was reduced slightly in each step of adsorption/regeneration cycle. Therefore, the FCMFs can be considered as a regenerable adsorbent.
 |
| | Fig. 12 Reusability of FCMFs for CV removal. Adsorbent: 200 mg L−1, pH: 7, dye concentration: 200 mg L−1, temperature: 293 K, contact time: 60 min. | |
Comparison with other adsorbents for the removal of CV
Several adsorbent materials were studied for removal of CV from aqueous solutions. Some of them are summarized in Table 6. The adsorbent used in present study showed excellent adsorbent capacity. As it can be observed, the adsorption capacity of FCMFs (872 mg g−1) is even significantly higher than that of non-environmentally friendly nanoadsorbents.
Table 6 The maximum adsorption capacities reported for adsorption of CV onto various adsorbents
| Adsorbent |
qm (mg g−1) |
Reference |
| Jute fiber carbon |
27.99 |
53 |
| Treated ginger waste (TGW) |
277.77 |
54 |
| Sulfuric acid activated carbon (SAAC) |
85.84 |
55 |
| Grapefruit peel |
254.16 |
56 |
| Jackfruit leaf powder (JLP) |
43.40 |
57 |
| NaOH-modified rice husk |
41.5 |
58 |
| Citric acid esterifying wheat straw (EWS) |
227.27 |
59 |
| Bacillus amyloliquefaciens biofilm |
582.41 |
60 |
| Modified rice husk |
93.45 |
61 |
| Carboxylate-functionalized sugarcane bagasse |
568.3 |
62 |
| Palm kernel fiber |
78.9 |
63 |
| Rice husk |
76.92 |
64 |
| Walnut shell |
90.8 |
65 |
| Chemically modified cellulose by anhydride |
370 |
66 |
| Functionalised multi-walled carbon nanotubes |
90.52 |
67 |
| CarAlg/MMt nanocomposite hydrogels |
88.8 |
68 |
| SnFe2O4@activated carbon magnetic nanocomposite |
158.73 |
69 |
| Magnetic nanocomposite |
113.31 |
70 |
| Cellulose |
2.42 |
71 |
| FCMFs |
872 |
Present study |
Conclusions
In this research, preparation of functionalized cellulosic microfibers (FCMFs) was proposed as a beneficial use of cellulosic healthcare wastes (CHCWs) for removal of crystal violet (CV) from aqueous solutions. Investigation of effective parameters showed that the adsorption efficiency was increased by increasing the salt content of wastewater. Therefore the adsorbent can be used successfully for treatment of textile industrial effluents which often contains high concentrations of electrolytes. The adsorption capacity was independent of pH in the range of 2–8 because the sulfate functional group is anionic in the wide range of pH. Therefore, variation of pH had no effect on the charge density of adsorbent. The effect of reaction time and CSA/cotton ratio on the charge density and adsorption capacity were investigated. The results showed that the charge density and adsorption capacity were increased by increasing the reaction time and the CSA/cotton ratio. The charge density of prepared FCMFs at the optimum condition was 2310 μeq. g−1 which is equivalent to 861 mg g−1. The equilibrium adsorption data were well described by Freundlich, Redlich–Peterson isotherms. The fitting degree followed the sequence: Freundlich = Radke–Prausnitz (best fit) > Redlich–Peterson > Dubinin–Radushkevich > Langmuir. The obtained maximum capacities using Langmuir, Dubinin–Radushkevich and Radke–Prausnitz models were 872, 837 and 714 mg g−1 respectively. The value of 1/nF was 0.035 which showed that the adsorption occurs through chemical interaction. The adsorption kinetic data were well fitted to pseudo-second-order and Elovich models and the fitting degree followed the sequence: pseudo-second-order (best fitting) > Elovich > intraparticle diffusion > liquid film diffusion > pseudo-first-order. Thermodynamic parameters indicated that the adsorption process was spontaneous and endothermic in nature. The saturated adsorbent was regenerated by acid washing (HCl, 1 mol L−1) and removal efficiency was decreased slightly in each batch adsorption/regeneration cycle.
Acknowledgements
The authors wish to acknowledge the Nanotechnology Research Center of Graduate Faculty of Environment, University of Tehran.
References
- A. Ahmad, S. H. Mohd-Setapar, C. S. Chuong, A. Khatoon, W. A. Wani, R. Kumar and M. Rafatullah, RSC Adv., 2015, 5, 30801–30818 RSC.
- R. Dutta, T. V Nagarjuna, S. A. Mandavgane and J. D. Ekhe, Ind. Eng. Chem. Res., 2014, 53, 18558–18567 CrossRef CAS.
- A. N. Kagalkar, R. V Khandare and S. P. Govindwar, RSC Adv., 2015, 5, 80505–80517 RSC.
- A. Mudhoo and D. Beekaroo, Adsorption of Reactive Red 158 Dye by Chemically Treated Cocos Nucifera L. Shell Powder: Adsorption of Reactive Red 158 by Cocos Nucifera L., Springer, Netherlands, 2011 Search PubMed.
- A. D. Scarfe, C. S. Lee and P. J. O'Bryen, Aquaculture Biosecurity: Prevention, Control, and Eradication of Aquatic Animal Disease, Wiley, 2006 Search PubMed.
- Y. Lin, X. He, G. Han, Q. Tian and W. Hu, J. Environ. Sci., 2011, 23, 2055–2062 CrossRef CAS.
- S. N. Singh, Microbial Degradation of Synthetic Dyes in Wastewaters, Springer International Publishing, 2014 Search PubMed.
- X. Zhang, W. Dong and W. Yang, Chem. Eng. J., 2013, 233, 14–23 CrossRef CAS.
- M. Cai, J. Su, Y. Zhu, X. Wei, M. Jin, H. Zhang, C. Dong and Z. Wei, Ultrason. Sonochem., 2016, 28, 302–310 CrossRef CAS PubMed.
- T. X. H. Le, M. Bechelany, S. Lacour, N. Oturan, M. A. Oturan and M. Cretin, Carbon, 2015, 94, 1003–1011 CrossRef CAS.
- E. M. Seftel, M. Niarchos, C. Mitropoulos, M. Mertens, E. F. Vansant and P. Cool, Catal. Today, 2015, 252, 120–127 CrossRef CAS.
- D. Dutta, D. Thakur and D. Bahadur, Chem. Eng. J., 2015, 281, 482–490 CrossRef CAS.
- X. Huang, B. Gao, Q. Yue, Y. Zhang and S. Sun, Sep. Purif. Technol., 2015, 154, 108–114 CrossRef CAS.
- B. Makhoukhi, M. A. Didi, H. Moulessehoul, A. Azzouz and D. Villemin, Appl. Clay Sci., 2010, 50, 354–361 CrossRef CAS.
- Q. Chen, P. Yu, W. Huang, S. Yu, M. Liu and C. Gao, J. Membr. Sci., 2015, 492, 312–321 CrossRef CAS.
- M. Punzi, A. Anbalagan, R. Aragão Börner, B.-M. Svensson, M. Jonstrup and B. Mattiasson, Chem. Eng. J., 2015, 270, 290–299 CrossRef CAS.
- M. T. Yagub, T. K. Sen, S. Afroze and H. M. Ang, Adv. Colloid Interface Sci., 2014, 209, 172–184 CrossRef CAS PubMed.
- V. K. Gupta, R. Kumar, A. Nayak, T. A. Saleh and M. A. Barakat, Adv. Colloid Interface Sci., 2013, 193–194, 24–34 CrossRef CAS PubMed.
- V. K. Gupta, S. Agarwal, P. Singh and D. Pathania, Carbohydr. Polym., 2013, 98, 1214–1221 CrossRef CAS PubMed.
- L. Gao, Y. Wang, T. Yan, L. Cui, L. Hu, L. Yan, Q. Wei and B. Du, New J. Chem., 2015, 39, 2908–2916 RSC.
- N. M. Mahmoodi, S. Khorramfar and F. Najafi, Desalination, 2011, 279, 61–68 CrossRef CAS.
- A. Elsaesser and C. V. Howard, Adv. Drug Delivery Rev., 2012, 64, 129–137 CrossRef CAS PubMed.
- D. Hristozov and I. Malsch, Sustainability, 2009, 1, 1161–1194 CrossRef CAS.
- I. Ali, M. Asim and T. A. Khan, J. Environ. Manage., 2012, 113, 170–183 CrossRef CAS PubMed.
- A. Pourjavadi, S. H. Hosseini, F. Seidi and R. Soleyman, Polym. Int., 2013, 62, 1038–1044 CrossRef CAS.
- H. Yan, H. Li, H. Yang, A. Li and R. Cheng, Chem. Eng. J., 2013, 223, 402–411 CrossRef CAS.
- H. Qiao, Y. Zhou, F. Yu, E. Wang, Y. Min, Q. Huang, L. Pang and T. Ma, Chemosphere, 2015, 141, 297–303 CrossRef CAS PubMed.
- A. A. Oladipo and M. Gazi, Journal of Water Process Engineering, 2014, 2, 43–52 CrossRef.
- M. Likhitha, R. R. N. Sailaja, V. S. Priyambika and M. V. Ravibabu, Int. J. Biol. Macromol., 2014, 65, 500–508 CrossRef CAS PubMed.
- G. R. Mahdavinia, F. Bazmizeynabad and B. Seyyedi, Desalin. Water Treat., 2013, 53, 2529–2539 CrossRef.
- S. Cui, S. Zhang, S. Ge, L. Xiong and Q. Sun, Ind. Crops Prod., 2016, 83, 346–352 CrossRef CAS.
- A. Prem Ananth, V. Prashanthini and C. Visvanathan, Waste Manag., 2010, 30, 154–161 CrossRef PubMed.
- T. Mitra, B. Singha, N. Bar and S. K. Das, J. Hazard. Mater., 2014, 273, 94–103 CrossRef CAS PubMed.
- Y. Xie, M. Wang and S. Yao, Langmuir, 2009, 25, 8999–9005 CrossRef CAS PubMed.
- L. Zhu, D. Lin and S. Yao, Carbohydr. Polym., 2010, 82, 323–328 CrossRef CAS.
- G. Chen, B. Zhang, J. Zhao and H. Chen, Food Hydrocolloids, 2014, 35, 476–483 CrossRef CAS.
- A. Kurniawan, H. Sutiono, N. Indraswati and S. Ismadji, Chem. Eng. J., 2012, 189–190, 264–274 CrossRef CAS.
- M. Nourani, M. Baghdadi, M. Javan and G. N. Bidhendi, Biochem. Pharmacol., 2016, 4, 1996–2003 CAS.
- S. D. Khattri and M. K. Singh, J. Hazard. Mater., 2009, 167, 1089–1094 CrossRef CAS PubMed.
- M. A. Ahmad and R. Alrozi, Chem. Eng. J., 2011, 171, 510–516 CrossRef CAS.
- T. Santhi, S. Manonmani and T. Smitha, J. Hazard. Mater., 2010, 179, 178–186 CrossRef CAS PubMed.
- M. Doğan, M. Alkan and Y. Onganer, Water, Air, Soil Pollut., 2000, 120, 229–248 CrossRef.
- B. H. Hameed, J. Hazard. Mater., 2009, 161, 753–759 CrossRef CAS PubMed.
- M.-H. Baek, C. O. Ijagbemi, O. Se-Jin and D.-S. Kim, J. Hazard. Mater., 2010, 176, 820–828 CrossRef CAS PubMed.
- K. Y. Foo and B. H. Hameed, Chem. Eng. J., 2010, 156, 2–10 CrossRef CAS.
- D. Mohan, K. P. Singh, G. Singh and K. Kumar, Ind. Eng. Chem. Res., 2002, 41, 3688–3695 CrossRef CAS.
- A. U. Itodo and H. U. Itodo, Life Sci. J., 2010, 7, 31–39 Search PubMed.
- N. K. Lazaridis and D. D. Asouhidou, Water Res., 2003, 37, 2875–2882 CrossRef CAS PubMed.
- R. Sivashankar, A. B. Sathya, K. Vasantharaj and V. Sivasubramanian, Environmental Nanotechnology, Monitoring and Management, 2014, 1–2, 36–49 CrossRef.
- M. Alkan, Ö. Demirbaş and M. Doğan, Microporous Mesoporous Mater., 2007, 101, 388–396 CrossRef CAS.
- J. C. Crittenden, R. R. Trussell, D. W. Hand, K. J. Howe and G. Tchobanoglous, MWH's Water Treatment: Principles and Design, John Wiley & Sons, 2012 Search PubMed.
- N. Kannan and G. Rengasamy, Water, Air, Soil Pollut., 2005, 163, 185–201 CrossRef CAS.
- K. Porkodi and K. Vasanth Kumar, J. Hazard. Mater., 2007, 143, 311–327 CrossRef CAS PubMed.
- R. Kumar and R. Ahmad, Desalination, 2011, 265, 112–118 CrossRef CAS.
- S. Senthilkumaar, P. Kalaamani and C. V. Subburaam, J. Hazard. Mater., 2006, 136, 800–808 CrossRef CAS PubMed.
- A. Saeed, M. Sharif and M. Iqbal, J. Hazard. Mater., 2010, 179, 564–572 CrossRef CAS PubMed.
- P. Das Saha, S. Chakraborty and S. Chowdhury, Colloids Surf., B, 2012, 92, 262–270 CrossRef CAS PubMed.
- S. Chakraborty, S. Chowdhury and P. Das Saha, Carbohydr. Polym., 2011, 86, 1533–1541 CrossRef.
- R. Gong, S. Zhu, D. Zhang, J. Chen, S. Ni and R. Guan, Desalination, 2008, 230, 220–228 CrossRef CAS.
- P. Sun, C. Hui, S. Wang, L. Wan, X. Zhang and Y. Zhao, Colloids Surf., B, 2016, 139, 164–170 CrossRef CAS PubMed.
- A. Masoumi, K. Hemmati and M. Ghaemy, Chemosphere, 2016, 146, 253–262 CrossRef CAS PubMed.
- B. C. S. Ferreira, F. S. Teodoro, A. B. Mageste, L. F. Gil, R. P. de Freitas and L. V. A. Gurgel, Ind. Crops Prod., 2014, 65, 521–534 CrossRef.
- G. O. El-Sayed, Desalination, 2011, 272, 225–232 CrossRef CAS.
- I. A. Rahman, B. Saad, S. Shaidan and E. S. S. Rizal, Bioresour. Technol., 2005, 96, 1578–1583 CrossRef CAS PubMed.
- M. K. Dahri, M. R. R. Kooh and L. B. L. Lim, J. Environ. Chem. Eng., 2014, 2, 1434–1444 CrossRef CAS.
- N. Gupta, A. K. Kushwaha and M. C. Chattopadhyaya, Arabian J. Chem., 2011 DOI:10.1016/j.arabjc.2011.07.021.
- V. Sabna, S. G. Thampi and S. Chandrakaran, Ecotoxicol. Environ. Saf., 2015 DOI:10.1016/j.ecoenv.2015.09.018.
- G. R. Mahdavinia, H. Aghaie, H. Sheykhloie, M. T. Vardini and H. Etemadi, Carbohydr. Polym., 2013, 98, 358–365 CrossRef CAS PubMed.
- P. Rai, R. K. Gautam, S. Banerjee, V. Rawat and M. C. Chattopadhyaya, J. Environ. Chem. Eng., 2015, 3, 2281–2291 CrossRef CAS.
- K. P. Singh, S. Gupta, A. K. Singh and S. Sinha, J. Hazard. Mater., 2011, 186, 1462–1473 CrossRef CAS PubMed.
- C. P. Sekhar, S. Kalidhasan, V. Rajesh and N. Rajesh, Chemosphere, 2009, 77, 842–847 CrossRef PubMed.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 









![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A + B1
A + B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln
qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qs − βε2
qs − βε2






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 865
865

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(Ke)
ln(Ke)











