Influence of alkyl chain branching point on the electron transport properties of di(perylene diimides) thin film transistors†
Abstract
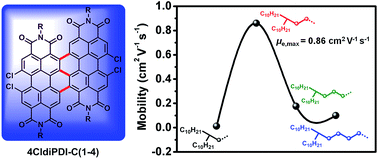
The alkyl chain length and density are fundamental factors affecting solution processability, molecular packing, film microstructure, and charge transport for organic thin film transistors (OTFTs). In this work, four tetra-chlorinated di(perylene diimides) 4CldiPDI-C(1–4) were prepared through moving the alkyl chain branching position away from the di(perylene diimides) backbone. OTFT devices employing 4CldiPDI-C(1–4) as the active layer were fabricated. Correspondingly, the effect of the position of the alkyl chain branching point on the film microstructure and charge transport were studied in detail. The research results demonstrate that the different branching point of side-chains has a negligible effect on the absorption maximum and energy gap. Conversely, the gradual movement in the branching point plays a key role in molecular packing and leads to a clear impact on electron mobility ranging from 0.012 to 0.86 cm2 V−1 s−1. Therefore, 4CldiPDI-C2-based devices offered the highest electron mobility of up to 0.86 cm2 V−1 s−1 and an on/off ratio of 2 × 107, which is among the best performance of diPDI derivatives.

 Please wait while we load your content...
Please wait while we load your content...