Electrochemical behavior of B(III) on a molybdenum electrode in NaCl–KCl eutectic molten salt
Qian Wangab,
Yan Li Wanga,
Hui Jun Liu*a and
Chao Liu Zenga
aLaboratory for Corrosion and Protection, Institute of Metal Research, Chinese Academy of Sciences, Shenyang, 110016, P R China. E-mail: liuhj@imr.ac.cn; Fax: +86-24-23904551; Tel: +86-24-23904553
bSchool of Materials Science and Technology, University of Science and Technology of China, Hefei, 230026, P R China
First published on 6th June 2016
Abstract
The electrochemical behavior of B(III) on a molybdenum electrode in NaCl–KCl–KBF4 molten salt at 1023 K is investigated and the diffusion coefficients of B(III) are calculated by cyclic voltammetry, square wave voltammetry and chronopotentiometry, respectively. The results show that the reduction of B(III) to B(0) is a diffusion-controlled single-step three-electron transfer reaction and the diffusion coefficient of B(III) is calculated to be 4.3 ± 0.4 × 10−5 cm2 s−1 at 1023 K by cyclic voltammetry. An individual spheroidal aggregate boron coating is obtained by galvanostatic electrolysis.
1. Introduction
The combination of both metal and nonmetal properties has made boron and its compounds attractive for practical applications in many fields.1–3 Boron fibers with high-tensile strength are used as reinforcements for both metals and plastics.4 Borides like refractory metal borides (TiB2, ZrB2, HfB2), B4C and BN have an integrated performance of sufficiently high thermal conductivity, good mechanical characteristics and excellent oxidation resistance, which can be used in aerospace industries.5 Besides, boron also finds its applications in metallurgy as deoxidizers and degasifiers.2There are several methods to prepare elemental boron from its compounds: (i) electrolysis from molten chloride or fluoride salts using borates or fluoroborates as boron source;6 (ii) metallic reduction using metals like Mg, Li, Na, K, Al, Fe;3 (iii) reduction of boron halides like BCl3 and BBr3 by hydrogen;7 (iv) thermal deposition of boron compounds.8 Among these methods, electrolysis in molten salts is considered as one of the most promising methods to prepare elemental boron due to its simple technical process, high purity of products and comparatively low cost.9
The preparation of boron by electrolysis in molten salts has been studied for nearly a century and different supporting electrolytes like chlorides, fluorides, mixed chlorides–fluorides and oxide–chlorides/fluorides, boron sources, electrolysis parameters have been compared to get the ideal products. Cooper et al.10 reported the electrolytic production of elemental boron in a KCl/KF–KBF4–B2O3 molten salt on different substrates like molybdenum, Inconel, low carbon iron at temperatures in the range from 923 to 1273 K. Ellis et al.11 studied the synthesis of boron from a HPO3–NaPO3–NH4BF4 (80 wt% HPO3, 15 wt% NaPO3 and 5 wt% NH4BF4) with 7 g B2O3 added as boron source and obtained boron at a rather low temperature of 523 K.
Wang et al.12 reported that the purities of the electrodeposited boron in three different molten salt systems followed in order by, mixed chloride–fluoride > chloride > fluoride. What's more, the chloride–fluoride molten salts are more promising as compared with fluoride and oxofluoride molten salts, which is mainly due to the reason that the former electrolyte system is less corrosive to the electrolysis equipment and the deposition products were easily separated from the residual salts. Besides, the current yield seems to be higher in chloride–fluoride melts than the other systems.13 As for the boron source, KBF4 and B2O3 are commonly applied in the preparation of boron. However, the purities of the products obtained from oxide-containing melts are comparatively low and tended to be contaminated by oxygen.14 Therefore, KBF4 is considered as a proper boron source.
Moreover, as we known, the mechanism of boron reduction in molten salt electrolysis is fundamental for improving the products' quality and purity. Taranenko et al.15 found that a three-electron cathodic reduction of BF4− was involved in a NaCl–KCl–MeBF4 (Me = Na, K) with the concentration range 3 × 10−5 to 80 × 10−5 mol cm−3 using a glass carbon electrode. Polyakova et al.16 studied the electrochemical behavior of boron reduction on glass carbon, Pt and Ag working electrodes in LiF–NaF–KF–KBF4 system at 823–973 K and came to the conclusion that B(III) was reduced to B in an irreversible three-electron reaction when the concentration of KBF4 was lower than 5.7 × 10−2 mol percent. When the concentration was higher than 5.7 × 10−2 mol percent, an ohmic resistance control obscured the kinetics of the cathode process. The cathodic processes of boron electrodeposition on a Pt electrode in LiF–KF–KBF4 and LiF–KF–B2O3 systems were investigated by Makyta et al.17 The LiF–KF–KBF4 system showed a similar three-electron reduction, while in the LiF–KF–B2O3 systems, B2O3 would firstly react with LiF and KF to form KBF4. Besides, the deposition potential difference of boron in these two systems is measured to be 0.47 V. Additionally, Rahul et al.18 studied the electrochemical deposition of boron on a Pt electrode from a KCl–KF–KBF4 system over the temperature range of 1073–1123 K, finding that the reversibility of the process was influenced by the scan rate. When the scan rate was below 0.1 V s−1, boron was reduced under a quasi-reversible single-step three-electron process. When it came to more than 0.1 V s−1, the reduction became irreversible.
From the above analysis, a NaCl–KCl–KBF4 molten salt system has been chosen to investigate the electrochemical behavior of B(III). Besides, the kinetic parameters like diffusion coefficients and diffusion activation energy are also calculated. These should be possible to gain some insight into the boron preparation in molten chloride–fluoride salts.
2. Experimental
2.1 Chemicals
The supporting electrolyte consists of a eutectic NaCl–KCl (supplied by Sinopharm Chemical Reagent Co., Ltd. analytical grade ≥99.5%) mixture with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mol%), which was dried under vacuum at 573 K for 48 h to remove residual water. Besides NaCl and KCl, dehydrated KBF4 (supplied by Shanghai SSS Reagent Co., Ltd. analytical grade ≥99.8%) was introduced into the electrolyte as the boron source to characterize the electrochemical behavior of boron and obtain the deposited boron.
1 (mol%), which was dried under vacuum at 573 K for 48 h to remove residual water. Besides NaCl and KCl, dehydrated KBF4 (supplied by Shanghai SSS Reagent Co., Ltd. analytical grade ≥99.8%) was introduced into the electrolyte as the boron source to characterize the electrochemical behavior of boron and obtain the deposited boron.
2.2 Electrochemical measurements
Fig. 1 shows the apparatus used in the experiment. The crucible was made of high purity graphite and placed in the bottom of a closed 201 stainless steel chamber, which was heated with well furnace under argon atmosphere with an argon flow rate of 5 mL min−1. The furnace was heated using a programmable furnace and the temperature was controlled by a PtRh–Pt thermocouple with an accuracy of ±1 K.All the electrochemical measurements were carried out by using a three-electrode system which was positioned in the graphite crucible as shown in Fig. 1. The reference electrode was a platinum wire (supplied by Rare Metallic Co., Ltd, 99.9% purity) and the surface area of the Pt electrode was kept constant for each experiment. Therefore, all potentials were referred to this Pt reference electrode unless otherwise stated. The graphite crucible was used as the counter electrode and a molybdenum wire (d = 1 mm, supplied by Rare Metallic Co., Ltd, 99.99% purity) served as the working electrode whose active electrode surface area was determined by measuring the immersion depth of the electrode in the melts. All the transient electrochemical measurements, including cyclic voltammetry, chronopotentiometry and square wave voltammetry, were performed with a PARSTAT 2273 electrochemical workstation.
2.3 Preparation of boron
The synthesis of boron in molten NaCl–KCl–KBF4 salt was carried out in a two-electrode system, using the graphite crucible as anode and stainless steel as cathode. The bulk 201 stainless steel was cut into specimens with a size of 20 mm × 10 mm × 2 mm by an electric spark cutting machine, ground down to 2000 grit SiC paper, rinsed in acetone by ultrasonic cleaning and then dried. Galvanostatic depositions of boron were finished at 1023 K and then the samples were pulled out of the molten salts and cooled in the air. Finally, the samples underwent a process of hot-water leaching, acid leaching, alcoholic washing and then drying.2.4 Characterization
The microstructure of the prepared boron was characterized by scanning electron microscope (SEM, FEI FP 2031/11 inspect F) coupled with an energy dispersive X-ray spectrometer (EDX).3. Results and discussion
3.1 Cyclic voltammograms of NaCl–KCl–KBF4 melt
Fig. 2 shows cyclic voltammograms of a molybdenum electrode (S = 0.314 cm2) in NaCl–KCl and NaCl–KCl–KBF4 molten salt electrolyte at 1023 K with different KBF4 concentrations. Form the insert, it can be seen a typical cyclic voltammogram recorded on a molybdenum electrode in NaCl–KCl eutectic molten salt electrolyte. Only cathodic peak C0 at −2.0 V vs. Pt and the corresponding anodic peak A0 at −1.8 V vs. Pt, corresponding to the deposition and dissolution of alkali metal, are observed within the defined electrochemical window. Furthermore, it can be observed that both of the cathodic and anodic peak currents increase with the increasing of KBF4 concentrations as shown in Fig. 2. Additionally, the cathodic peak potentials move to more negative direction with the increasing of the KBF4 concentration suggesting that the electrode process is not completely reversible. By comparison with the three curves, curve 2 reveals better cyclic voltammetry behavior of B(III) ion on a molybdenum electrode and more information could be obtained than the other two curves. Therefore, a NaCl–KCl eutectic molten salt with the KBF4 concentration of 3.177 × 10−4 mol cm−3 was chosen in the following investigation.The cyclic voltammograms of B(III) ion on a molybdenum electrode in NaCl–KCl–KBF4 (3.177 × 10−4 mol cm−3) molten salt electrolyte at 1023 K with different reversing potentials at a scan rate of 0.5 V s−1 are shown in Fig. 3. The four curves reveal that one cathodic peak and three anodic peaks are obtained for different reversing potentials. The cathodic peak C1 at −0.9 V vs. Pt and the corresponding anodic peak A1, A2, and A3, respectively, are corresponding to the reduction of B(III) ion to B(0) and the oxidation of B(0) to B(III), as shown in Fig. 3. The cathodic peaks show that the electrochemical reduction of B(III) ion is a single-step process, while the three anodic peaks reveal the oxidation of B(0) to B(III) is a three-step process. In addition, the cathodic peak currents and the anodic peak currents both increase with the reversing potential shifts from −1.2 to −1.5 V. However, the increments of anodic peak currents are larger than those of cathodic peak currents, which may be attributed to the reason that more B(III) ion is reduced to boron when more negative potentials are applied, thus more boron is to be oxidized on the positive direction scanning.
Fig. 4 reveals the cyclic voltammograms of a molybdenum electrode in NaCl–KCl–KBF4 (3.177 × 10−4 mol cm−3) molten salt electrolyte at 1023 K at different scan rates. It shows that the cathodic peaks move to more negative potential with the increasing scan rates. Furthermore, the potential difference between cathodic peak and anodic peak, ΔEp(ΔEp = |ECp − EAp|),19 is larger than the value of 0.068 V for a three-electron reaction at 1023 K calculated by the eqn (1), revealing that the electrochemical process for the deposition/dissolution reaction of B is not a completely reversible process.
 | (1) |
 | ||
| Fig. 4 Cyclic voltammograms of a molybdenum electrode (S = 0.314 cm2) in NaCl–KCl–KBF4 (3.177 × 10−4 mol cm−3) molten salt electrolyte with various scan rates. Reference electrode: Pt. T = 1023 K. | ||
Table 1 shows the parameters obtained from cyclic voltammograms in Fig. 4. It can be seen that the value of iCp/iAp are gradually deviating from 1 with the increasing scan rates which indicates that the reversibility of the reaction in low scan rate is better than that of high scan rate. In other words, the process is moving towards irreversible with the increasing scan rates. This is in agreement with the results obtained by Rahul et al.18 that the process is quasi-reversible (scan rate < 0.1 V s−1) and irreversible (scan rate > 0.1 V s−1).
| Scan rate (v/V s−1) | Cathodic peak potential (ECp/V) | Anodic peak potential (EAp/V) | Cathodic peak current (iCp/A) | Anodic peak current (iAp/A) | Cathodic half peak potential (ECp/2/V) | iCp/iAp |
|---|---|---|---|---|---|---|
| 0.3 | −0.943 | −0.574 | −0.050 | 0.041 | −0.877 | 1.22 |
| 0.4 | −0.957 | −0.611 | −0.062 | 0.046 | −0.891 | 1.35 |
| 0.6 | −0.965 | −0.660 | −0.073 | 0.052 | −0.898 | 1.42 |
| 0.7 | −0.971 | −0.596 | −0.080 | 0.063 | −0.906 | 1.28 |
| 0.8 | −0.977 | −0.612 | −0.088 | 0.062 | −0.910 | 1.41 |
| 1 | −0.983 | −0.675 | −0.091 | 0.063 | −0.917 | 1.45 |
Fig. 5 shows the cyclic voltammograms of a molybdenum electrode in NaCl–KCl–KBF4 (3.177 × 10−4 mol cm−3) molten salt electrolyte at 973 K at different scan rates. It is observed that both of the cathodic peak current and anodic peak current at 973 K are lower than those at 1023 K at the same scan rate by comparison with Fig. 5 and 4, which may be due to the reason that the diffusion rate of B(III) ion increases with temperature.
 | ||
| Fig. 5 Cyclic voltammograms of a molybdenum electrode (S = 0.314 cm2) in NaCl–KCl–KBF4 (3.177 × 10−4 mol cm−3) molten salt electrolyte with various scan rates. Reference electrode: Pt. T = 973 K. | ||
To further investigate the reversibility of the electrode reaction, the relationship between ECp and logarithm of scan rate is plotted according to Fig. 4 and shown in Fig. 6. It reveals linear relationship between logarithm of scan rate and ECp, which further confirms the electrochemical process for the deposition/dissolution reaction of B is a quasi-reversible reaction.
The diffusion coefficient of B(III) can also be calculated using the following method with the data from Fig. 6. For an irreversible reaction, the peak current, iP, can be expressed as the following Delahay equation:20
 | (2) |
The  in eqn (2) can be obtained from the slope of Fig. 6 according to the following equation:21,22
in eqn (2) can be obtained from the slope of Fig. 6 according to the following equation:21,22
 | (3) |
Therefore, the diffusion coefficient of the B(III) at 1023 K can be calculated by eqn (2) and the result is 4.3 ± 0.4 × 10−5 cm2 s−1 at 1023 K. Then, in a similar way, the diffusion coefficient of the B(III) at 973 K can be also obtained as 2.9 ± 0.2 × 10−5 cm2 s−1 according to Fig. 5. This further illustrates that both of the cathodic peak current and anodic peak current at 1023 K are larger than that at 973 K at the same scan rate as shown in Fig. 4 and 5, respectively.
The relationship between the cathodic peak current and the square root of the scan rate is plotted in Fig. 7. It can be seen that the cathodic peak current is directly proportional to the square root of the scan rate, revealing that the electrochemical reduction of B(III) on molybdenum electrode is a diffusion-controlled process.
 | ||
| Fig. 7 Linear relationship of the peak current for B(III) reduction versus square root of the scan rate. | ||
It is the fact that the working electrode used in the experiment is in a cylindrical shape and the immersion depth in the molten salt is far larger than its diameter. Therefore, the diffusion along the cross section of the electrode can be neglected and the diffusion along the radial direction is just considered, which makes the discussion of the diffusion current more convenient. Based on this hypothesis, the linear relationship between Ep and  can be obtained as the following equation:23
can be obtained as the following equation:23
 | (4) |
The cathode current values on different potentials can be obtained from the cyclic voltammograms on various scan rates and the plots of E versus  are shown in Fig. 8. The slope of the equation can be obtained through making linear fitting of the plots. Then the number of the transfer electron for the reduction of B(III) ion can be calculated from the slope of the linear plots and listed in Table 2. It can be seen that the transfer electron numbers calculated based on different scan rates are all close to 3, which further proves that the electrochemical reduction of B(III) ion on a molybdenum electrode in the NaCl–KCl–KBF4 system is a diffusion-controlled single-step three-electron transfer reaction, shown as following:
are shown in Fig. 8. The slope of the equation can be obtained through making linear fitting of the plots. Then the number of the transfer electron for the reduction of B(III) ion can be calculated from the slope of the linear plots and listed in Table 2. It can be seen that the transfer electron numbers calculated based on different scan rates are all close to 3, which further proves that the electrochemical reduction of B(III) ion on a molybdenum electrode in the NaCl–KCl–KBF4 system is a diffusion-controlled single-step three-electron transfer reaction, shown as following:
| B(III) + 3e− → B | (5) |
| Scan rate (v/V s−1) | Slope from linear fitting | Calculated transfer electron number/n |
|---|---|---|
| 0.4 | −0.0346 | 2.6 |
| 0.6 | −0.0273 | 3.2 |
| 0.8 | −0.0323 | 2.7 |
| 1 | −0.0332 | 2.7 |
3.2 Square wave voltammetry
To further confirm the transferred electrons of the electrochemical reduction of B(III) ion, Square Wave Voltammetry (SWV) is applied to study the electrochemical reduction of B(III) in the NaCl–KCl–KBF4 molten salt electrolyte at 1023 K and the result is shown in Fig. 9. It shows that a good symmetric wave at −0.94 V (vs. Pt) is obtained, which agrees with the value of the cathodic peak potential in CV curves as shown in Fig. 3. Therefore, the transferred electrons of the electrochemical reduction of B(III) ion can also be calculated by following equation:19
 | (6) |
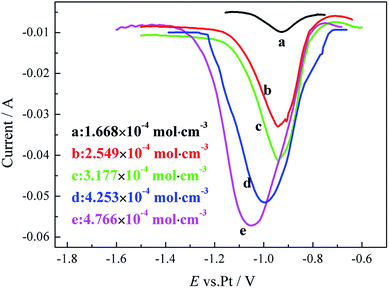
Fig. 10 represents square wave voltammograms of a molybdenum electrode (S = 0.314 cm2) in NaCl–KCl–KBF4 molten salt electrolyte at 1023 K with different KBF4 concentrations. It can be seen that the reduction peak currents increase with the KBF4 concentrations. Besides, according to the report of Chamelot,24 the reduction peak current of the square wave voltammogram is formally expressed as follows:
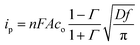 | (7) |
 | (8) |
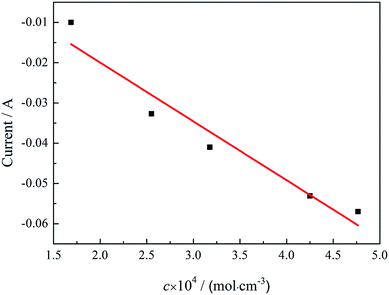
The relationship between the reduction peak current ip and the concentration of KBF4 in the melts is plotted and represented in Fig. 11. It can be seen that the peak current varies linearly with the concentration which is consistent with eqn (7). From the data extracted from square wave voltammograms and using eqn (7) and (8), the diffusion coefficient of KBF4 in the melt at the temperature of 1023 K can also be calculated to be 3.4 ± 0.2 × 10−5 cm2 s−1.
3.3 Chronopotentiometry
To further confirm the mechanism of the electrochemical reduction of B(III) ion, chronopotentiometry is applied by using a molybdenum electrode in NaCl–KCl–KBF4 (3.177 × 10−4 mol cm−3) molten salt electrolyte at 1023 K at different currents and the results are shown in Fig. 12. One plateau in low current range (10–20 mA) while two plateaus are observed in high current range (30–50 mA). Combining with the results of cyclic voltammogram as shown in Fig. 3, it can be deduced that the first plateau at −0.8 to −1.0 V is corresponding to the deposition of boron and the second plateau at −1.4 to −1.8 V is referred to the deposition of alkali metal. | ||
| Fig. 12 Chronopotentiograms of a molybdenum electrode (S = 0.314 cm2) in the NaCl–KCl–KBF4 (3.177 × 10−4 mol cm−3) molten salt electrolyte at 1023 K at different currents. | ||
The plot of the current versus the reciprocal square root of the transition time is obtained and shown in Fig. 13. According to Sand equation as shown in eqn (9) (ref. 19) and combining with the results from chronopotentiograms, the average diffusion coefficient of B(III) can be calculated to be 5.2 ± 0.5 × 10−5 cm2 s−1, which is in agreement with the results calculated by eqn (2).
 | (9) |
In addition, the influence of temperature on the diffusion coefficient of B(III) was also determined by plotting ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D versus the reverse of absolute temperature. The diffusion activation energy can be calculated using the following equation:
D versus the reverse of absolute temperature. The diffusion activation energy can be calculated using the following equation:
 | (10) |
From this equation, the diffusion activation energy is calculated to be 65.2 kJ mol−1.
Table 3 summarizes the diffusion coefficient of B(III) ion obtained by several researches and the results of this work by electrochemical methods. The results show that the diffusion coefficients of B(III) estimated in this work accord with the data obtained from other researchers in the same magnitude. There are many factors that may influence the diffusion coefficient like temperature, the different molten salt systems and complexion behavior between the ions. As stated by Kuznetsov,26 the following reaction may happen during the process which leads to the formation of complexion of boron and fluoride/chloride ion:
| BF4− + Cl− ⇌ BF3Cl− + F− | (11) |
| Methods | Diffusion coefficient (D × 105/cm2 s−1) | Molten salt | Sources | ||
|---|---|---|---|---|---|
| 973 K | 1023 K | 1073 K | |||
| CV (Delahay eqn) | 2.9 ± 0.2 | 4.3 ± 0.4 | — | NaCl–KCl | This study |
| Square wave voltammetry | — | 3.4 ± 0.2 | — | NaCl–KCl | This study |
| Chronopotentiometry | — | 5.2 ± 0.5 | — | NaCl–KCl | This study |
| CV (Delahay eqn) | — | — | 2.5 ± 0.4 | KCl–KF | 18 |
| Chronoamperometry | — | — | 1.2 ± 0.3 | KCl–KF | 18 |
| CV (Delahay eqn) | 2.1 | — | — | LiF–NaF–KF | 16 |
| Chronopotentiometry | 4.4 | — | — | LiF–KF | 17 |
| CV (Delahay eqn) | — | 2.4 | — | NaCl–KCl–NaF | 25 |
Therefore, the concentration of BF4− and fluoride/chloride ion can affect the equilibrium of the upper reaction, which will have an influence on the diffusion coefficient of B(III).
3.4 Electrodeposition and characterization of boron
According to the above discussion, the boron is obtained on stainless steel plate by galvanostatic electrolysis with a constant current of 0.3 A for 0.5 h in NaCl–KCl–KBF4 molten salt. The SEM image and EDX analysis of the electrodeposited boron is shown in Fig. 14. The results show that the prepared boron is composed of an individual spheroidal with the average size of 200 nm. EDX analysis conforms that the deposition is mainly composed of boron, more than 97%, and minor impurities which may come from the remained salt. It is reported by Cooper6 and Nies27 that the purities of the boron prepared from KCl–KBF4–B2O3 and KCl–KF–B2O3 systems, respectively, were varied from 90–97% and 93.7–97%. Therefore, the purity of the obtained boron is considerable comparing with these literature, which confirms that the NaCl–KCl–KBF4 molten salt system is feasible for the production of high-purity boron.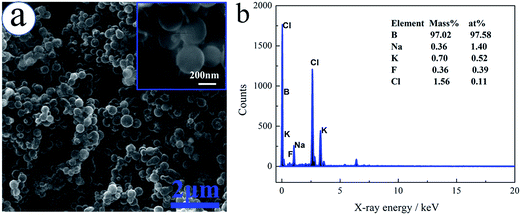 | ||
| Fig. 14 SEM image and EDX analysis of the electrodeposited boron in NaCl–KCl–KBF4 (3.177 × 10−4 mol cm−3) molten salt with a constant current of 0.3 A for 0.5 h. | ||
4. Conclusions
The reduction of B(III) to B(0) on a molybdenum electrode in NaCl–KCl–KBF4 eutectic molten salt at 1023 K is a diffusion-controlled single-step three-electron transfer reaction. The diffusion coefficient of B(III) is calculated to be 2.9 ± 0.2 × 10−5 cm2 s−1 at 973 K and 4.3 ± 0.4 × 10−5 cm2 s−1 at 1023 K by cyclic voltammetry. The diffusion activation energy is calculated to be 65.2 kJ mol−1. In addition, an individual spheroidal aggregate boron coating with an average size of 200 nm is obtained on a stainless steel substrate by galvanostatic electrolysis.Acknowledgements
This work is supported by National Natural Science Foundation of China Grant No. 51271190 and 51501205.References
- V. I. Matkovich, Boron and refractory borides, Springer-Verlag, New York, 1976 Search PubMed.
- W. G. Woods, Environ. Health Perspect., 1994, 102(suppl. 7), 5–11 CrossRef CAS PubMed.
- N. N. Greenwood and A. Earnshaw, Chemistry of the elements, Butterworth-Heinemann, 1998 Search PubMed.
- P. M. R. Ricceri, Int. J. Powder Metall., 2003, 39, 48–52 Search PubMed.
- E. P. Simonenko, D. V. Sevast’yanov, N. P. Simonenko, V. G. Sevast’yanov and N. T. Kuznetsov, Russ. J. Inorg. Chem., 2013, 58, 1669–1693 CrossRef CAS.
- H. S. Cooper, US Pat., 2,572,249, 1951.
- D. T. Hurd, J. Am. Chem. Soc., 2002, 71, 20–22 CrossRef.
- D. K. Das and K. Kumar, Thin Solid Films, 1981, 83, 53–60 CrossRef CAS.
- V. V. Malyshev, Russian Journal of Non-Ferrous Metals, 2007, 48, 177–183 CrossRef.
- H. S. Cooper, US Pat., 2,984,605, 1961.
- R. B. Ellis, US Pat., 2,810,683, 1957.
- Z. Wang, Z. Qiu, Y. Yu and Y. Peng, Non-ferrous Min, Metall., 1998, 14, 23–26 Search PubMed.
- S. V. Devyatkin, G. Kaptay, V. I. Shapoval, I. V. Zarutskii, V. P. Lugovoi and S. A. Kuznetsov, Nato Arw on Refractory Metals in Molten Salts, 1997, vol. 53, pp. 73–80 Search PubMed.
- V. I. Shapoval, I. V. Zarutskii, V. V. Malyshev and N. N. Uskova, Russ. Chem. Rev., 1999, 68, 925–933 CrossRef CAS.
- V. I. Taranenko, I. V. Zarutskii, V. I. Shapoval, M. Makyta and K. Matiašovský, Electrochim. Acta, 1992, 37, 263–268 CrossRef.
- L. P. Polyakova, J. Electrochem. Soc., 1996, 143, 3178–3191 CrossRef CAS.
- M. Makyta, K. Matiašovský and P. Fellner, Electrochim. Acta, 1984, 29, 1653–1657 CrossRef CAS.
- R. Pal, S. Anthonysamy and V. Ganesan, J. Electrochem. Soc., 2012, 159, 157–165 CrossRef.
- A. J. Bard, Electrochemical Methods, Fundamentals & Applications, 2001 Search PubMed.
- P. Delahay and C. N. Reilley, New instrumental methods in electrochemistry : theory, instrumentation, and applications to analytical and physical chemistry, R. E. Krieger Pub. Co., 1980 Search PubMed.
- S. A. Kuznetsov, H. Hayashi, K. Minato and M. Gaune-Escard, J. Electrochem. Soc., 2005, 152, 203–212 CrossRef.
- S. A. Kuznetsov, H. Hayashi, K. Minato and M. Gaune-Escard, Electrochim. Acta, 2006, 51, 2463–2470 CrossRef CAS.
- E. Wang and Z. Zhang, Electrochemical Principles and Methods, Science Press, Beijing, 2000 Search PubMed.
- P. Chamelot, B. Lafage and P. Taxil, Electrochim. Acta, 1998, 43, 607–616 CrossRef CAS.
- G. A. Bukatova, S. A. Kuznetsov and M. Gauneescard, J. Min. Metall., Sect. B, 2003, 39, 251–259 CrossRef CAS.
- S. A. Kuznetsov, Russ. J. Electrochem., 1996, 32, 763–769 CAS.
- N. P. Nies, J. Electrochem. Soc., 1960, 107, 817–820 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |