Stoichiometry-controlled cycloaddition of nitrilimines with unsymmetrical exocyclic dienones: microwave-assisted synthesis of novel mono- and dispiropyrazoline derivatives†
Houda Gazzeha,
Sarra Boudrigaa,
Moheddine Askri*a,
Abderrahim Khatyrb,
Michael Knorrb,
Carsten Strohmannc,
Christopher Golzc,
Yoann Rousselind and
Marek M. Kubickid
aLaboratory of Heterocyclic Chemistry Natural Product and Reactivity/CHPNR, Department of Chemistry, Faculty of Science of Monastir, 5000 Monastir, Tunisia. E-mail: Moheddine.Askri@fsm.rnu.tn
bInstitut UTINAM – UMR CNRS 6213, Université de Franche-Comté, 16 Route de Gray, 25030 Besançon, France
cTechnische Universität Dortmund, Anorganische Chemie Otto-Hahn-Strasse 6, 44221 Dortmund, Germany
dInstitute of Molecular Chemistry – UMR CNRS 6302, University of Bourgogne, 9 Avenue A. Savary, F-21078 Dijon, France
First published on 11th May 2016
Abstract
Microwave-assisted 1,3-dipolar cycloaddition of (E,E)-1,3-bisarylidenetetral-2-ones with nitrilimines, generated in situ by dehydrohalogenation of the corresponding hydrazonoyl chlorides, affords a series of spiropyrazolines in good to excellent yields. The presence of a second exocyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond also allows the preparation of dispiropyrazolines. The cycloaddition proceeds with high chemo-, regio- and diastereoselectivity. The structure of the spiranic adducts has been established on the basis of their spectroscopic data and elemental analyses. The stereochemistry of these N-heterocycles has been confirmed by two X-ray diffraction studies. The luminescence properties and fluorescence quantum yields of these heterocyclic compounds have been investigated revealing that some of them are fluorescent in solution.
C bond also allows the preparation of dispiropyrazolines. The cycloaddition proceeds with high chemo-, regio- and diastereoselectivity. The structure of the spiranic adducts has been established on the basis of their spectroscopic data and elemental analyses. The stereochemistry of these N-heterocycles has been confirmed by two X-ray diffraction studies. The luminescence properties and fluorescence quantum yields of these heterocyclic compounds have been investigated revealing that some of them are fluorescent in solution.
Introduction
Pyrazolines are fascinating scaffolds which constitute the core structure of a wide range of medicinally relevant compounds exhibiting numerous important bioactivities including anti-inflammatory,1 antimicrobial,2 anticancer,3 antitubercular,4 antiproliferative,5 antidepressant6 and antifungal properties7 (Fig. 1). Moreover, pyrazoline derivatives represent an important class of organic materials of great significance widely used as fluorescent probes8 and materials9 due to their high hole-transport efficiency, strong fluorescence and excellent blue-emitting properties with high quantum yield.10 These nitrogen-heterocycles have also found industrial applications, for example, they are used as whitening and brightening agents for optical bleaching of paper, plastics and textiles.11The significance of the pyrazoline architecture with regard to their wide range of biological and photophysical properties has stimulated a great demand for their efficient synthetic methods.12 Among the numerous developed strategies, the 1,3-dipolar cycloaddition reaction of nitrilimines with alkenes has been proven to be a robust method to build up the pyrazoline scaffold in a highly regio- and stereoselectivity fashion.13 Thereby, exocyclic enones have been occasionally applied as dipolarophiles for the synthesis of spiropyrazoline derivatives (Scheme 1).14 However, a survey of the literature reveals that there are only two examples by A. S. Girgis and co-workers15 describing symmetrical exocyclic dienones as the reaction partner with nitrilimines (Scheme 1). However, these cycloadditions occur with relative low chemoselectively, providing a mixture of mono-and dispiropyrazoline products.
 | ||
| Scheme 1 Profile of 1,3-dipolar cycloadditions for exocyclic enones and dienones across nitrilimines. | ||
In light of the limited study of these interesting bis-exocyclic double bond systems, we were prompted to investigate the chemical activity of unsymmetrical exocyclic dienones for [3 + 2] cycloaddition reactions (Scheme 1), not reported till to date.
Thus, it appeared to be highly desirable to explore this kind of reaction which could constitute a promising strategy for the synthesis of diverse mono- and dispiropyrazolines.
In view of the challenge using unsymmetrical exocyclic dienones as dipolarophiles and in continuation of our ongoing investigation on the synthesis of spiroheterocycles,16 we herein report the first microwave-assisted chemo-, regio- and diastereoselective 1,3-dipolar cycloaddition between (E,E)-1,3-bisarylidenetetral-2-ones and in situ generated nitrilimines stemming from their corresponding hydrazonoyl chlorides. This reaction provides an efficient and practical access to mono- and dispiro-containing pyrazoline cores with hitherto unreported substitution pattern. Furthermore, we demonstrate the role of stoichiometry-controlled cycloaddition in modulating the chemoselectivity of the 1,3-dipolar cycloaddition reaction. Finally, we report on the luminescence properties of some selected heterocyclic compounds of this family.
Results and discussion
Synthesis of mono- and dispiropyrazolines
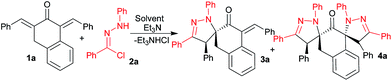
The dienones chosen for our study as dipolarophiles have been prepared by the acid-catalysed condensation of β-tetralone with various benzaldehydes. As confirmed by NMR spectroscopy, these alkenes display an (E,E)-configuration in accordance with the literature data.17 These dipolarophiles have two activated exocylic double bonds. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond at the 3-position is expected to be more reactive than the second double bond due to the reduced steric hindrance (Fig. 2). To prove this hypothesis, we chose to study at the onset the reaction between (E,E)-1,3-bisbenzylidenetetral-2-one 1a and the nitrilimine generated in situ by dehydrohalogenation of the corresponding hydrazonoyl chloride 2a in 1
C double bond at the 3-position is expected to be more reactive than the second double bond due to the reduced steric hindrance (Fig. 2). To prove this hypothesis, we chose to study at the onset the reaction between (E,E)-1,3-bisbenzylidenetetral-2-one 1a and the nitrilimine generated in situ by dehydrohalogenation of the corresponding hydrazonoyl chloride 2a in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 molar ratio (Scheme 2).
1.5 molar ratio (Scheme 2).
In order to optimize the reaction conditions, a range of solvents with different polarities such as toluene, acetonitrile and dichloromethane were explored (Table 1).
| Entry | Solvent | Conditions | Ratio 3a/4a | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (1 mmol), 2a (1.5 mmol), Et3N (3 mmol), solvent (5 mL).b Product ratios were determined by analysis of the 1H NMR spectrum of the crude reaction mixture.c Isolated yield after purification by column chromatography on silica gel.d Inseparable mixture of products. | ||||
| 1 | PhCH3 | rt, 76 h | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
32 |
| 2 | PhCH3 | 110 °C, 24 h | — | —d |
| 3 | CH3CN | rt, 73 h | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
35 |
| 4 | CH3CN | 82 °C, 24 h | — | —d |
| 5 | CH2Cl2 | rt, 48 h | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20b 20b |
54/8 |
| 6 | CH2Cl2 | MW (100 W, 50 °C, 10 m) | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10b 10b |
75/12 |
| 7 | CH2Cl2 | MW (100 W, 60 °C, 15 m) | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10b 10b |
78/5 |
| 8 | CH2Cl2 | MW (100 W, 80 °C, 15 m) | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10b 10b |
85/7 |
As shown in Table 1, when carrying out the reaction in toluene or acetonitrile at room temperature, the desired product 3a is obtained with poor yield (Table 1, entries 1 and 3). Raising the reaction temperature to reflux for 24 h led to an inseparable mixture of products (Table 1, entries 2 and 4). Performing in dichloromethane at room temperature, the reaction proceeded smoothly affording a mixture of monospiropyrazoline 3a and dispiropyrazoline 4a in a 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio (Table 1, entry 5) with a moderate yield (Table 1, entry 5). Next, the reaction was examined under microwave irradiation (Table 1, entries 6–8). Under this condition, an enhanced reactivity and high selectivity compared to the above reactions was noticed. The best results are observed at 80 °C to afford the pyrazoline 3a in excellent yield (85%) within 15 min (Table 1, entry 8). The drastic reduction in the reaction time with enhanced yield, high chemo- and regioselectivity makes this strategy the most advantageous.
20 ratio (Table 1, entry 5) with a moderate yield (Table 1, entry 5). Next, the reaction was examined under microwave irradiation (Table 1, entries 6–8). Under this condition, an enhanced reactivity and high selectivity compared to the above reactions was noticed. The best results are observed at 80 °C to afford the pyrazoline 3a in excellent yield (85%) within 15 min (Table 1, entry 8). The drastic reduction in the reaction time with enhanced yield, high chemo- and regioselectivity makes this strategy the most advantageous.
To address a possible influence of the electronic propensity of the substituent at the para-position of the aryl group of dipolarophiles and dipoles-1,3 on the yield and regiochemical outcome of the reaction, a variety of dienones 1a–f and hydrazonoyl chlorides 2a, b with both electron-donating and electron-withdrawing substituent were treated under the optimized conditions (Scheme 3 and Table 2).
 | ||
| Scheme 3 1,3-Dipolar microwave-assisted cycloaddition reactions for the synthesis of monospiropyrazoline derivatives 3. | ||
| Entry | Compounds | Ar | Ar′ | 1/2 with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 molar ratio (reaction time 15 m) 1.5 molar ratio (reaction time 15 m) |
1/2 with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio (reaction time 30 m) 3 molar ratio (reaction time 30 m) |
||
|---|---|---|---|---|---|---|---|
| Ratio 3/4a | Yieldb (%) | Ratio 3/4a | Yieldb (%) | ||||
| a The relative ratios were determined by analysis of the 1H NMR spectra of the crude reaction mixture.b Isolated yield after purification by column chromatography on silica gel. | |||||||
| 1 | 3a/4a | Ph | Ph | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
85/7 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
10/75 |
| 2 | 3b/4b | p-MeC6H4 | Ph | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
73/14 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 86 |
18/73 |
| 3 | 3c/4c | p-MeOC6H4 | Ph | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
82/14 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83 83 |
14/82 |
| 4 | 3d/4d | p-ClC6H4 | Ph | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
77/11 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
11/77 |
| 5 | 3e/4e | p-CNC6H4 | Ph | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
83/13 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 87 |
10/73 |
| 6 | 3f/4f | Ph | p-MeC6H4 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
73/10 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82 82 |
13/70 |
| 7 | 3g/4g | p-MeC6H4 | p-MeC6H4 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
70/13 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
14/77 |
| 8 | 3h/4h | p-MeOC6H4 | p-MeC6H4 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
77/14 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
10/75 |
| 9 | 3i/4i | p-ClC6H4 | p-MeC6H4 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
75/10 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 84 84 |
11/72 |
| 10 | 3j/4j | p-CNC6H4 | p-MeC6H4 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
72/11 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 85 |
15/79 |
As shown in Table 2, the reaction proceeds with high chemoselectivity as it occurs preferentially on the exocyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond at 3-position of dipolarophiles 1, affording the mono-spiropyrazolines 3 as the major product. This reaction also proceeds with high regioselectivity with the carbon of the dipole adding to the β-position of C
C double bond at 3-position of dipolarophiles 1, affording the mono-spiropyrazolines 3 as the major product. This reaction also proceeds with high regioselectivity with the carbon of the dipole adding to the β-position of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. The resulting adducts were obtained as single diastereoisomers (Scheme 3). Similar tendency has been recently observed in other 1,3-dipolar cycloadditions involving EWG-substituted ethylene derivatives.18 The yields of the produced pyrazolines 3 were marginally affected by the substituent present on the aromatic ring of the dienones and 1,3-dipoles (Table 2).
C double bond. The resulting adducts were obtained as single diastereoisomers (Scheme 3). Similar tendency has been recently observed in other 1,3-dipolar cycloadditions involving EWG-substituted ethylene derivatives.18 The yields of the produced pyrazolines 3 were marginally affected by the substituent present on the aromatic ring of the dienones and 1,3-dipoles (Table 2).
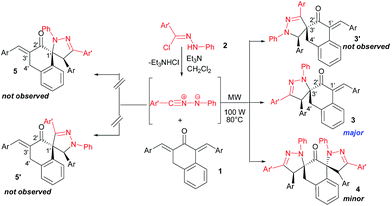
Next, we explored the reaction under similar conditions with one equivalent of dipolarophiles 1 and three equivalents of hydrazonoyl chloride 2, precursor of the in situ generated 1,3-dipole, under microwave irradiation (Scheme 4 and Table 2).
Interestingly, upon running the reaction for 30 min and increasing the temperature to 120 °C, the chemoselectivity of the reaction is modulated and we obtained the dispiropyrazolines 4 as the major product with good yields (Table 2).
Spectroscopic and crystallographic characterization of the mono- and dispiropyrazoline derivatives
The structure and the relative configuration of the mono- and dispiropyrazoline were deduced from their spectroscopic data and from two X-ray structure determinations performed on cycloadducts 3b and 4c. Selected chemical shift of pyrazolines 3b and 4c are depicted in Fig. 3 and 4, respectively.The 1H NMR spectrum of compound 3b (Fig. 3) reveals two mutually coupled doublets at δ 2.76 ppm and 3.93 ppm (J = 15.3 Hz) corresponding to the H-4′ methylene protons proving the chemoselectivity of the cycloaddition reaction. If the hypothetical alternative isomers 5 or 5′ (Scheme 3) would have been formed, the H-4'protons should give rise to a doublet. The appearance of the singlet at δ 4.47 is consistent with the pyrazoline-4H proton (typically δ 4.7–5.1)15,19 and not with the pyrazoline-5H proton in 3′b (Scheme 3). The latter should resonate at a δ value >5.6 ppm.20
In addition, the 13C NMR spectrum exhibits a signal at δ 75.2 ppm attributed to spiranic carbon C-5 in accordance with the literature.21 Therefore, these results data confirm the existence of regioisomer 3b and rule out the alternative structure 3′b (Scheme 3).
The 1H NMR spectrum of compounds 4c show two singlets at δ 4.86 and 5.01 ppm corresponding to pyrazolinic protons H-4 and H-4′ indicating the formation of two pyrazolinic rings. The geminal protons H-4′′ give rise to two doublets at 3.25 ppm and 3.43 ppm. Of particular significance is the absence of signals at about 7.19 ppm corresponding to an olefinic proton. Furthermore, the 13C NMR spectrum of compound 4c displays two signals at 73.9 ppm and 83.3 ppm corresponding to two spiranic carbons C-5 and C-5′, which is close to analogous reported simple structures.21 These data support the pyrazoline structure 4 and rule out the formation of isomeric compound 4′ (Scheme 4).
Single-crystal X-ray diffraction analysis of 3b (Fig. 5) confirmed the regio-isomeric structure assigned to this product, and indicated the retention of primary E-configuration of diplarophile which confirms one-step cycloaddition mechanism.22 Moreover, it precises that the molecule presented in Fig. 5 (from asymmetric unit) has a (1S, 7S) configuration. Since the space group of crystallization of 3b (P21/c) bears the symmetry centres, the second enantiomer (R, R) is also present in the lattice. The relative configuration of this dispiropyrazoline was assigned as (9S, 10S) and (7R, 15R) for the enantiomer of cycloadduct 4c shown in Fig. 6 (note the labelling of the carbon atoms for the X-ray structures is different from that used for assignment of the NMR data). The examination of this structure reveals a cis-relationship between the N-phenyl of the nitrilimine part and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the tetralone ring. Thus, the cycloadduct 4 is formed via the approach of the dipole from the bottom side of the C
O group of the tetralone ring. Thus, the cycloadduct 4 is formed via the approach of the dipole from the bottom side of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of cycloadduct 3.
C double bond of cycloadduct 3.
This approach should be more favourable, whereas the formation of the other diastereoisomer 6 is less favourable due to steric repulsion between the phenyl groups of the tetralone and the N-phenyl rings (Fig. 7). This, in turn, also explains the facial diastereoselectivity encountered during the second cycloaddition. These findings show again that this 1,3-dipolar cycloaddition occurs with interesting molecular diversity and high diastereoselectivity.
Electronic properties
The absorption and emission spectral data obtained for compounds 3 and 4 are summarized in Table 3.| Compound | λabs (nm) (log(ε) M−1 cm−1) | λexcitb (nm) | λemc (nm) | ϕd (%) |
|---|---|---|---|---|
| a Values of absorption maximum (λabs.) and their associated molar extinction coefficient (ε).b λexcit.: excitation wavelength.c λem.: emission wavelength.d ϕ: fluorescence quantum yield (±8%). | ||||
| 3a | 240 (4.21); 341 (4.16) | 350 | 453 | 0.67 |
| 3b | 234 (4.34); 356 (4.32) | 330 | 407 (sh); 429 | 1.20 |
| 3c | 237 (4.23); 356 (4.21) | 350 | 454 | 0.88 |
| 3d | 240 (4.21); 349 (4.19) | 350 | 427 | 0.91 |
| 3f | 243 (4.23); 347 (4.22) | 350 | 434 | 0.76 |
| 3g | 245 (4.22); 351 (4.23) | 350 | 410 | 0.97 |
| 3h | 249 (4.21); 355 (4.20) | 350 | 427 | 0.74 |
| 3i | 251 (4.19); 347 (4.17) | 340 | 403 | 0.82 |
| 3j | 245 (4.26); 335 (4.04) | 340 | 452 | 1.04 |
| 4a | 239 (4.56); 349 (4.37) | 330 | 408 | 1.47 |
| 4b | 232 (4.61); 346 (4.29) | 350 | 420 | 1.68 |
| 4c | 244 (4.60); 347 (4.37) | 330 | 402 | 1.52 |
All absorption spectra have a similar appearance. For comparison, the superposition of the electronic absorption spectra of representative compounds 3b and 4b measured with a concentration around 2 × 10−5 M at 298 K using dichloromethane as solvent are shown in Fig. 8.
However, one can note that the absorption intensities between the two bands around 234 and 356 nm of the two compounds are different. Increasing the number of phenyl groups in compound 4b in comparison with 3b results an increase of the molar coefficients extinction of the bands at strong energy (Table 3). These two absorption bands can be attributed to transitions between π, π* states, whereas the very broad absorption bands around 370 nm are due to a mixture of π, π* and n, π* characters.
The superposition of the emission spectra of compounds 3b and 4b (ca. 1.2 × 10−6 M) measured at 298 K in degassed dichloromethane are shown in Fig. 9. After excitation of compounds 3b and 4b at 330 nm and 350 nm, the emission maxima are observed at 407 and 429 nm, respectively.
The emission spectra have a low symmetry like that of the band to weak energy of the absorption spectra (Fig. 9).
Both measurements recorded in the presence of oxygen or the increase of the concentration lead to similar emission spectra. These findings allow to exclude a deactivation from a triplet state or an emission stemming from a excimer in solution. Indeed, the broad fluorescence bands observed for 3b and 4b can safely be assigned to a π, π* state, it follows that the absorption transition is belonging to a higher π, π* state or to a state different character, e.g. a charge transfer state. Either way, the lowest energy singlet state S1 → S0 (the one seen in fluorescence) must be hidden beneath the more intense S2 → S0 band.23 Fluorescence quantum yields ØF of compounds 3 (0.67 to 1.2) and 4 (1.47 to 1.68) were determined using cresyl violet as a fluorescence quantum yield standard.24 Generally, these values are relatively low compared with other spiro-compounds reported in the literature,24 but follow a similar trend reported for some other molecules of this family.25
Conclusion
The microwave-assisted 1,3-dipolar cycloaddition of nitrilimine with (E,E)-1,3-bisarylidenetetral-2-ones provides to be a straightforward entry to mono- and dispiropyrazolines. The reaction proceeds with high chemo-, regio- and diastereoselectivity, affording adducts with high to excellent yields. The study of the luminescence properties of spiropyrazolines 3 and 4 revealed that some of them are fluorescent in solution. Because of the unique structure of the pyrazolines and the easiness and advantages of this microwave-assisted protocol, such as greater reactivity, mild condition, and high selectivity and a broad substrate scope, we believe that this method could be very useful for drug discovery and development. Therefore, the biological activities of our spiropyrazoline derivatives deserve investigation. Further research is also in progress to extend the scope of these reactions and to analyze/understand theoretically the approach between dipole and dipolarophile by means of DFT computing. Notably the possibility to construct novel heterocyclic systems by cycloaddition of other dipoles such as organic azides RN3 on the remaining reactive exocyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of 3 appears as an interesting objective for forthcoming studies.
C bond of 3 appears as an interesting objective for forthcoming studies.
Experimental
General methods
NMR spectra were recorded with a Bruker-Spectrospin AC 300 spectrometer operating at 300 MHz for 1H and 75 MHz for 13C relative to TMS as internal standard. CDCl3 was used as solvent. Splitting patterns are designated as follows: s, singlet; d, doublet; m, multiplet. Chemical shift (δ) values are given in parts per million and coupling constants (J) in Hertz. IR spectra were recorded on a Perkin-Elmer Spectrum TwoTM FT-IR in the ATR mode. Melting points were determined by using an Electro-thermal 9100 digital melting point apparatus. Materials: thin layer chromatography TLC plates (Merck, Silca gel 60 F254 0.2 mm 200 × 200 mm) substances were detected using UV-light at 254 nm. UV-vis spectra were measured with a VARIAN-Cary 100 spectrophotometer and emission spectra were recorded on a Jobin-Yvon Fluoro Log 3.2.2 spectrofluorometer. Small-scale microwave-assisted synthesis was carried out in a StartSynth multimode microwave instrument producing controlled irradiation at 2.45 GHz (Milestone S.r.l., Sorisole, Italy). The instrument is equipped with an industrial magnetron and a microwave diffuser located above the microwave chamber, with continuous microwave output power from 0 to 1400 W. Reaction times refer to hold times at the temperatures indicated, not to total irradiation times. The synthesis of hydrazonoyl chlorides was accomplished by reacting the corresponding N-phenyl-N′-arylhydrazines with triphenylphosphine and carbon tetrachloride in acetonitrile at room temperature following the procedure of A. S. Shawali et al.26General procedure for the synthesis of spiropyrazoline derivatives 3
A mixture of 1,3-bis(arylidene)-2-tetralones 1 (1 mmol), hydrazonoyl chloride (1.5 eq.) 2 and Et3N (3 eq.) in dichloromethane (5 mL), was stirred in a microwave reactor at 80 °C for 15 m. Then the mixture was washed with a brine solution and extracted three times with dichloromethane. The organic layer was dried over MgSO4 and concentrated in vaccuo. The spiroadducts were separated by using column chromatography on silica gel using petroleum ether/acetone (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent, and then crystallized from ethanol.
2) as eluent, and then crystallized from ethanol.
General procedure for the synthesis of spiropyrazoline derivatives 4
Compounds 4 were obtained under comparable reaction conditions regardless of the stoichiometry of hydrazonoyl chloride (3 eq.) 2 and NEt3 (6 eq.). Spectroscopic data for all compound are presented in the ESI.†X-ray structure studies
A colourless single-crystal of 3b has been mounted on a Nonius Kappa Apex II diffractometer and the intensity data have been collected at 115 K with MoKα radiation (λ 0.71073 Å). These data were further treated with the SAINT V8.27B program suite (Bruker AXS Inc., 2012) and within the OLEX2 frame.27 The structure has been solved by direct methods with SHELXS-97 and refined using SHELXL-97.28 There is a disorder over two positions of C-9 through C-14 phenyl ring bound to the C8 atom of the main molecular core of 3b. The occupancies of both sites have been refined to some 0.7 for the major structure (A) and to 0.3 for a minor phenyl (B). This disorder of one phenyl ring in the crystal of 3b has no influence on the stereochemistry of the molecule.The crystal structure determinations of 4c was accomplished on an Oxford Diffraction Xcalibur Sapphire 3 diffractometer; data collection: CrysAlis CCD (Oxford Diffraction, 2013); cell refinement: CrysAlis RED (Oxford Diffraction, 2013); data reduction: CrysAlis RED; absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2013). The structure was solved by applying direct methods (ShelXS) and refined with ShelXL28 using OLEX2.27 Furthermore, the crystal structure of 4c contains disordered solvent molecules which were removed from the model using the solvent mask function of OLEX2.29
All non-hydrogen atoms in both structures were refined anisotropically. The hydrogen atoms where introduced within a riding model and refined with isotropic temperature factors set to Uiso(H) = 1.5Ueq.(C) for hydrogen atoms on sp3 carbons and to Uiso(H) = 1.2Ueq.(C) for those ridden on sp2 carbons.
Crystallographic data (excluding structure factors) for the structures of 3b and 4c have been deposited at CCDC with deposition number (1412290 and 1471593).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943 reflections collected, 7201 unique and 5049 with I > 2σ(I). Final agreement factors: R(F) = 0.1095 (all reflections) and 0.0757 with I > 2σ(I). Final residuals ρmax = 0.637, ρmin = −0.379 e− Å−3. GOF = 1.024.
943 reflections collected, 7201 unique and 5049 with I > 2σ(I). Final agreement factors: R(F) = 0.1095 (all reflections) and 0.0757 with I > 2σ(I). Final residuals ρmax = 0.637, ρmin = −0.379 e− Å−3. GOF = 1.024.Acknowledgements
We thank Dr Kabula Ciamala for helpful discussion. M. K. and M. M. K. thank the CNRS for financial support.References
- (a) J. He, L. Ma, Z. Wei, J. Zhu, F. Peng, M. Shao, L. Lei, L. He, M. Tang, L. He, Y. Wu and L. Chen, Bioorg. Med. Chem. Lett., 2015, 25, 2429–2433 CrossRef CAS PubMed; (b) R. Aggarwal, A. Bansal, I. Rozas, B. Kelly, P. Kaushik and D. Kaushik, Eur. J. Med. Chem., 2013, 70, 350–357 CrossRef CAS PubMed; (c) S. Y. Jadhav, S. P. Shirame, S. D. Kulkarni, S. B. Patil, S. K. Pasale and R. B. Bhosale, Bioorg. Med. Chem. Lett., 2013, 23, 2575–2578 CrossRef CAS PubMed.
- (a) S. Bano, M. S. Alam, K. Javed, M. Dudeja, A. K. Das and A. Dhulap, Eur. J. Med. Chem., 2015, 95, 96–103 CrossRef CAS PubMed; (b) S. S. Sulthana, S. A. Antony, C. Balachandran and S. S. Shafi, Bioorg. Med. Chem. Lett., 2015, 25, 2753–2757 CrossRef PubMed; (c) P. F. Wang, H. Y. Qiu, J. T. Ma, X. Q Yan, H. B. Gong, Z. C. Wang and H. L. Zhu, RSC Adv., 2015, 5, 24997–25005 RSC; (d) F. B. Abdel-Wahab, A. H. Abdel-Aziz and E. M. Ahmed, Eur. J. Med. Chem., 2009, 44, 2632–2635 CrossRef PubMed; (e) A. Wael, E. Sayed, I. F. Nassar, A. H. Adel and A. Rahman, Monatsh. Chem., 2009, 140, 365–370 CrossRef.
- (a) H. L. Qin, Z. P. Shang, I. Jantan, O. U. Tan, M. A. Hussain, M. Sherd and S. N. A. Bukhari, RSC Adv., 2015, 5, 46330–46338 RSC; (b) A. Monteiro, L. M. Gonçalves and M. M. Santos, Eur. J. Med. Chem., 2014, 79, 266–272 CrossRef CAS PubMed; (c) S. K. Kankala, R. K. Kankala, D. R. Kommidi, C. Mudithanapelli, R. Balaboina, R. Vadde, S. B. Jonnalagadda and C. S. Vasam, RSC Adv., 2014, 4, 40305–40311 RSC; (d) V. K. Rao, R. Tiwari, B. S. Chhikara, A. N. Shirazi, K. Parang and A. Kumar, RSC Adv., 2013, 3, 15396–15403 RSC.
- (a) M. J. Ahsan and V. Sainib, Beni-Suef University Journal of Basic and Applied Sciences, 2015, 4, 41–46 CrossRef; (b) A. Ahmad, A. Husain, S. A. Khan, M. Mujeeb and A. Bhandari, J. Saudi Chem. Soc., 2014 DOI:10.1016/j.jscs.2014.12.004; (c) M. G. Mamolo, D. Zampieri, V. Falagiani, L. Vio and E. Banfi, Farmaco, 2001, 56, 593–599 CrossRef CAS PubMed.
- (a) P. Rathore, S. Yaseen, S. Ovais, R. Bashir, R. Yaseen, A. D. Hameed, M. Samim, R. Gupta, F. Hussain and K. Javed, Bioorg. Med. Chem. Lett., 2014, 24, 1685–1691 CrossRef CAS PubMed; (b) F. M. Awadallah, G. A. Piazza, B. D. Gary, A. B. Keeton and J. C. Canzoneri, Eur. J. Med. Chem., 2013, 70, 273–279 CrossRef CAS PubMed.
- (a) N. Gokhan-Kelekçi, S. Koyunoglu, S. Yabanoglu, K. Yelekci, O. Ozgen, G. Uçar, K. Erol, E. Kendi and A. Yesilada, Bioorg. Med. Chem., 2009, 17, 675–689 CrossRef PubMed; (b) O. D. Can, U. D. Ozkay, Z. A. Kaplancikli and Y. Ozturk, Arch. Pharmacal Res., 2009, 32, 1293–1299 CrossRef CAS PubMed.
- (a) I. Ali, W. A. Wani, A. Khan, A. Haque, A. Ahmad, K. Saleem and N. Manzoor, Microb. Pathog., 2012, 53, 66–73 CrossRef CAS PubMed; (b) A. A. Bekhit and T. Abdel-Aziem, Bioorg. Med. Chem., 2004, 12, 1935–1945 CrossRef CAS PubMed.
- (a) X. X. Zheng, S. Q. Wang, H. Y. Wang, R. R. Zhang, J. T. Liu and B. X. Zhao, Spectrochim. Acta, Part A, 2015, 138, 247–251 CrossRef CAS PubMed; (b) X. Dai, T. Zhang, Y. Z. Liu, T. Yan, Y. Li, J. Y Miao and B. X. Zhao, Sens. Actuators, B, 2015, 207, 872–877 CrossRef CAS; (c) A. Joseph Cotruvo Jr, A. T. Aron, K. M. Ramos-Torres and J. C. Chang, Chem. Soc. Rev., 2015, 44, 4400–4414 RSC; (d) Y. Liu, S. Q Wang and B. X. Zhao, RSC Adv., 2015, 5, 32962–32966 RSC; (e) F. Yu, X. Hana and L. Chen, Chem. Commun., 2014, 50, 12234 RSC; (f) T. T. Zhang, X. P. Chen, J. T. Liu, L. Z. Zhang, J. M. Chu, L. Su and B. X. Zhao, RSC Adv., 2014, 4, 16973–16978 RSC; (g) M. Verma, A. F. Chaudhry, M. T. Morgan and C. J. Fahrni, Org. Biomol. Chem., 2010, 8, 363–370 RSC.
- (a) V. Ramkumar and P. Kannan, Opt. Mater., 2015, 46, 314–323 CrossRef CAS; (b) D. Yan and D. G. Evans, Mater. Horiz., 2014, 1, 46–57 RSC; (c) S. P. Anthony, ChemPlusChem, 2012, 77, 518–531 CrossRef CAS; (d) B. Bian, S. J. Ji and H. B. Shi, Dyes Pigm., 2008, 76, 348–352 CrossRef CAS.
- (a) A. Abbas, N. Flores-Holguin and M. M. Naseer, New J. Chem., 2015, 39, 4359–4367 RSC; (b) A. Abbas, S. Hussain, N. Hafeez, A. Hasan and M. M. Naseer, Spectrochim. Acta, Part A, 2014, 127, 32–40 CrossRef CAS PubMed; (c) B. Dong, M. Wang and C. Xu, Luminescence, 2013, 28, 628–633 CrossRef CAS PubMed; (d) S. Q. Wang, Q. H. Wu, H. Y. Wang, X. X. Zheng, S. L. Shen, Y. R. Zhang, J. Y. Miao and B. X. Zhao, Analyst, 2013, 138, 7169–7174 RSC; (e) J. F. Li, D. X. Li, Y. Y. Han, S. M. Shuang and C. Dong, Spectrochim. Acta, Part A, 2009, 73, 221–225 CrossRef PubMed; (f) J. Ji and H. B. Shi, Dyes Pigm., 2006, 70, 246–250 CrossRef; (g) C. J. Fahrni, L. C. Yang and D. G. Van-Derveer, J. Am. Chem. Soc., 2003, 125, 3799–3812 CrossRef CAS PubMed.
- (a) J. N. Yao, D. B. Xiao, X. Lu, W. S. Yang, H. B. Fu, Z. G. Shuai and Y. Fang, J. Am. Chem. Soc., 2003, 125, 6740–6745 CrossRef PubMed; (b) A. Wagner, C. W. Schellhammer and P. S. Petersen, Angew. Chem., Int. Ed., 1966, 5, 699–704 CrossRef CAS; (c) F. Wilkinson, G. P. Kelly, C. Michael and D. Oelkrug, J. Photochem. Photobiol., A, 1990, 52, 309–443 CrossRef CAS; (d) J. Pan, U. Scherf, A. Schreiber, R. Bilke and D. Haarer, Synth. Met., 2000, 115, 79–82 CrossRef CAS; (e) S. Tasch, A. Niko, G. Leising and U. Scherf, Appl. Phys. Lett., 1996, 68, 1090–1092 CrossRef CAS; (f) M. UChida, Y. Ohmori, C. Morishima and K. Yoshino, Synth. Met., 1993, 57, 4168–4173 CrossRef CAS.
- (a) Y. Suzuki, S. Naoe, S. Oishi, N. Fujii and H. Ohno, Org. Lett., 2012, 14, 326–329 CrossRef CAS PubMed; (b) Q. Wu, P. Liu, Y.-M. Pan, Y.-L. Xu and H.-S. Wang, RSC Adv., 2012, 2, 10167–10170 RSC; (c) S. Fustero, M. Sánchez-Roselló, P. Barrio and A. Simón-Fuentes, Chem. Rev., 2011, 111, 6984–7034 CrossRef CAS PubMed; (d) X.-H. Liu, B.-F. Ruan, J. Li, F.-H. Chen, B.-A. Song, H.-L. Zhu, P. S. Bhadury and J. Zhao, Mini-Rev. Med. Chem., 2011, 11, 771–821 CrossRef CAS PubMed; (e) X.-H. Liu, B.-F. Ruan, J.-X. Liu, B.-A. Song, L.-H. Jing, J. Li, Y. Yang, H.-L. Zhu and X.-B. Qi, Bioorg. Med. Chem. Lett., 2011, 21, 2916–2920 CrossRef CAS PubMed; (f) T. Okitsu, K. Sato and A. Wada, Org. Lett., 2010, 12, 3506–3509 CrossRef CAS PubMed.
- (a) G. Wang, X.-H. Liu, T. Huang, Y.-L. Kuang, L. Lin and X.-M. Feng, Org. Lett., 2013, 15, 1 CrossRef PubMed; (b) A. Singh, A. L. Loomer and G. P. Roth, Org. Lett., 2012, 14, 5266–5269 CrossRef CAS PubMed; (c) T. Hashimoto and K. Maruoka, in Handbook of Cyclization Reactions, ed. S. Ma, Wiley-VCH, Weinheim, 2009, ch. 3, pp. 87–168 Search PubMed; (d) G. Molteni, Heterocycles, 2005, 65, 2513–2537 CrossRef CAS; (e) G. Broggini, I. De Marchi, M. Martinelli, G. Paladino, T. Pilati and A. Terraneo, Synthesis, 2005, 13, 2246–2252 CrossRef; (f) P. D. Buttero, G. Molteni and T. Pilati, Tetrahedron, 2005, 61, 2413–2419 CrossRef; (g) Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry-Toward Heterocycles and Natural Products, ed. A. Padwa and W. H. Pearson, Wiley, Hoboken, 2003 Search PubMed; (h) K. V. Gothelf, in Cycloaddition Reactions in Organic Synthesis, ed. S. Kobayashi and K. A. Jørgensen, Wiley-VCH, Weinheim, 2002, vol. 25, pp. 21–247 Search PubMed; (i) K. V. Gothelf and K. A. Jørgensen, Chem. Rev., 1998, 98, 863–909 CrossRef CAS PubMed.
- (a) L. A. Gerten, M. C. Slade, K. M. Pugh and L. M. Stanley, Org. Biomol. Chem., 2013, 11, 7834–7837 RSC; (b) K. M. Dawood, Tetrahedron, 2005, 61, 5229–5233 CrossRef CAS; (c) A. Kerbal, K. Tshiamala, J. Vebrel, B. Laude and M. F. Mercier, Bull. Soc. Chim. Belg., 1991, 100, 159–168 CrossRef CAS; (d) E. Jedlovska, A. Levai, G. Toth, B. Balazs and L. Fisera, J. Heterocycl. Chem., 1999, 36, 1087–1090 CrossRef CAS; (e) N. Mishriky, A. S. Girgis and Y. A. Ibrahim, J. Chem. Res., 2000, 1, 101–111 Search PubMed.
- (a) A. S. Girgis, F. F. Barsoumb and A. Samir, Eur. J. Med. Chem., 2009, 44, 1–5 CrossRef PubMed; (b) A. S. Girgis, Y. A. Ibrahim, N. Mishriky, J. N. Lisgarten, B. S. Pottercand and R. A. Palmer, Tetrahedron, 2001, 57, 2015–2019 CrossRef CAS.
- (a) C. Mhiri, S. Boudriga, M. Askri, M. Knorr, D. Sriram, P. Yogeeswari, F. Nana, K. Golz and C. Strohmanni, Bioorg. Med. Chem. Lett., 2015, 25, 4308–4313 CrossRef CAS PubMed; (b) S. Haddad, S. Boudriga, F. Parzo, A. Soldera, M. Askri, M. Knorr, Y. Rousselin, M. M. Kubicki, K. Golz and C. Strohmann, J. Org. Chem., 2015, 80, 9064–9075 CrossRef CAS PubMed; (c) C. Mhiri, F. Rouatbi, S. Boudriga, M. Askri, K. Ciamala, M. Knorr, K. Monnier-Jobé, A. Khatyr, Y. Rousselin and M. M. Kubicki, Mediterr. J. Chem., 2015, 4, 30–50 CrossRef; (d) F. Rouatbi, M. Askri, F. Nana, G. Kirsch, S. Sriram and P. Yogeeswari, Tetrahedron Lett., 2015, 57, 163–167 CrossRef; (e) S. Haddad, S. Boudriga, F. Parzo, A. Soldera, M. Askri, D. Sriram, P. Yogeeswari, M. Knorr, Y. Rousselin and M. M. Kubicki, RSC Adv., 2014, 4, 59462–59471 RSC.
- J. R. Dimmock and M. P. Padmanilyama, Eur. J. Med. Chem., 2002, 37, 813–824 CrossRef CAS PubMed.
- (a) R. Jasinski, M. Ziokowska, O. M. Demchuk and A. Maziarka, Cent. Eur. J. Chem., 2014, 12, 586–593 CrossRef CAS; (b) R. Jasinski, O. Koifman and A. Baranski, Mendeleev Commun., 2011, 21, 262–263 CrossRef CAS.
- (a) H. Behbehani, H. M. Ibrahim and K. M. Dawood, RSC Adv., 2015, 5, 25642–25649 RSC; (b) A. L. Gerten, M. l C. Slade, K. M. Pugh and L. i M. Stanley, Org. Biomol. Chem., 2013, 11, 7834–7837 RSC; (c) A. Singh, A. L. Loomer and G. P. Roth, Org. Lett., 2012, 14, 5266–5269 CrossRef CAS PubMed; (d) A. S. Girgis, Y. A. Ibrahim, I. S. Ahmed-Farag and N. Mishriky, J. Chem. Res., 2000, 11, 508–509 CrossRef; (e) R. Raghunathan, M. Shanmugasundaram, S. Bhanumathi and P. J. E. Malar, Heteroat. Chem., 1998, 9, 327–332 CrossRef CAS; (f) T. Fathi, K. Ciamala, N. Dinh An and J. Vebrel, Can. J. Chem., 1994, 72, 1424–1428 CrossRef CAS.
- H. M. Hassaneen, R. H. Hilal, N. M. Elwan, A. Harhash and A. S. Shawali, J. Heterocycl. Chem., 1984, 21, 1013–1016 CrossRef CAS.
- (a) A. S. Girgis, Y. A. Ibrahim, N. Mishriky, J. N. Lisgarten, B. S. Potter and R. A. Palmer, Tetrahedron, 2001, 57, 2015–2019 CrossRef CAS; (b) A. S. Girgis, J. Chem. Res., 2006, 3, 127–129 CrossRef.
- (a) R. Jasinski, RSC Adv., 2015, 5, 101045–101048 RSC; (b) R. Jasinski, Tetrahedron Lett., 2015, 56, 532–535 CrossRef CAS.
- (a) J. B. Birks, Photophysics of Aromatic Molecules, Wiley-Interscience, New York, 1970 Search PubMed; (b) A. Khatyr and R. Ziessel, J. Org. Chem., 2000, 65, 3126–3134 CrossRef CAS PubMed.
- (a) D. Magde, J. H. Brannon, T. L. Cremers and J. Olmsted, J. Phys. Chem., 1979, 83, 696–699 CrossRef CAS; (b) H. Maas, A. Khatyr and G. Calzaferri, Microporous Mesoporous Mater., 2003, 65, 233–242 CrossRef CAS.
- (a) A. Kundu, S. Pathak and A. Pramanik, Asian J. Org. Chem., 2013, 2, 869–876 CrossRef CAS; (b) K. Nasu, T. Nakagawa, H. Nomura, C.-J. Lin, C.-H. Cheng, M.-R. Tseng, T. Yasuda and C. Adachi, Chem. Commun., 2013, 49, 10385–10387 RSC.
- A. S. Shawali, H. M. Hassaneen and H. A. Ibrahim, Arch. Pharmacal Res., 1990, 13, 126–131 CrossRef CAS.
- OLEX2: a complete structure solution, refinement and analysis program. O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- B. Rees, L. Jenner and M. Yusupov, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2005, 61, 1299–1301 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1412290 and 1471593. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra09703k |
| This journal is © The Royal Society of Chemistry 2016 |