Heterogeneous Nb-containing catalyst/N,N-dimethylacetamide–salt mixtures: novel and efficient catalytic systems for the dehydration of fructose†
Zhimin Xue*a,
Bobo Caob,
Wancheng Zhaoc,
Jinfang Wangc,
Tingting Yuc and
Tiancheng Mu*c
aBeijing Key Laboratory of Lignocellulosic Chemistry, College of Materials Science and Technology, Beijing Forestry University, Beijing 100083, P. R. China. E-mail: zmxue@bjfu.edu.cn
bSchool of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, P. R. China
cDepartment of Chemistry, Renmin University of China, Beijing 100872, P. R. China. E-mail: tcmu@ruc.edu.cn; Tel: +86-10-62514925
First published on 28th June 2016
Abstract
The development of efficient catalytic systems for the dehydration of carbohydrates to produce 5-hydroxymethylfurfural (HMF) is a very attractive topic. In this work, we synthesized a novel Nb-containing catalyst by the reaction of niobium chloride and nitrilotris(methylenephosphonic acid) (NTMPA), denoted as Nb–NTMPA. The synthesized Nb–NTMPA was characterized by powder X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), N2 adsorption–desorption, and Fourier transform infrared spectroscopy and used as the heterogeneous catalyst for the dehydration of fructose into HMF using the mixture of N,N-dimethylacetamide (DMA) and salts as the reaction solvent. It was found that Nb–NTMPA was very active for the reaction and a HMF yield of 85.6% could be achieved in a DMA–NaBr mixture under the optimal reaction conditions. Further study indicated that the salts could affect the activity of the reaction systems by the formation of DMA·M+ (M = Li, Na and K) macrocations and weakly ion-paired halide ions. Moreover, Nb–NTMPA/DMA–salt systems could also be used in the production of HMF from inulin and sucrose with satisfactory yields.
Introduction
Conversion of renewable and abundant biomass resources into liquid fuels and value-added chemicals has attracted much attention in recent years.1–5 Among various processes explored for biomass transformation, dehydration of carbohydrates, an important type of biomass, to produce 5-hydroxymethylfurfural (HMF) is one of the most successful routes.6–9 As one of the top building block compounds obtained from biomass,10 HMF can be transformed into many kinds of value-added compounds and materials,11–14 which are currently produced mainly from petroleum. In the production of HMF from carbohydrates, the dehydration of fructose is the most promising and efficient method.Up to now, various catalysts, including homogeneous and heterogeneous catalysts, have been developed for the dehydration of fructose to produce HMF. Homogeneous catalysts include mineral or organic acids,15 various Lewis acids,16,17 and ionic liquids.18–21 Generally, these homogeneous catalysts are effective for the dehydration. But the separation and recovery of the catalysts are difficult, which limit the wide application of these catalysts. In order to overcome the separation problem, numerous heterogeneous catalysts have been designed for the dehydration of fructose, such as acid resins,22,23 tin-beta zeolite,24 lignosulfonic acid25 sulfated zirconia,26 Nb-containing compounds,27 sulfonated carbon,28,29 graphene oxide,30 supported ionic liquids,31,32 zirconium phosphonate,33 metal–organic frameworks,34 and Fe3O4–SBA–SO3H,35 etc. Regardless of these developments, it remains to be a hot topic to obtain HMF from fructose over heterogeneous catalysts efficiently.
Among the above catalysts, Nb-containing compounds are an important type of heterogeneous catalysts for the dehydration of fructose into HMF because of their high stability and strong acid properties. For example, Nb2O5 has been proved to be an effective catalyst for fructose dehydration in DMSO and a HMF yield of 86.2% could be achieved at 120 °C with a reaction time of 2 h.36 Niobic acid and niobium phosphate could also be used as catalysts for the dehydration of fructose to produce HMF in the aqueous phase or water/2-butanol biphasic system.37 It was found that niobium phosphate had higher catalytic activity than niobic acid, which was resulted from the higher effective acidity on the surface of niobium phosphate. Moreover, sulfated mesoporous niobium oxide showed a much higher activity for the dehydration than commercial Nb2O5 because of its higher surface area and acidity.38 Recently, a new kind of mesoporous niobium phosphates was synthesized and exhibited excellent activity in the dehydration of fructose to HMF in water.39 These achievements of Nb-containing compounds provide an attractive way for designing new heterogeneous Nb-containing catalysts for the production of HMF from fructose.
Except for catalysts, solvents also have important effect on the activity and selectivity for the formation of HMF from fructose. The dehydration of fructose could be conducted in various solvents systems, including traditional organic solvents,40,41 water,42 multiphase systems,43,44 and ionic liquids.45 Since alkylimidazolium chloride ionic liquids were found to be an efficient solvent for dehydration of carbohydrates into HMF, ionic liquids as the dehydration solvents have attracted dramatic attention. However, the expensive price of ionic liquids limited its large-scale application. Therefore, seeking new solvents is still a very attractive topic in the formation of HMF from the dehydration of fructose. Recent reports indicated that N,N-dimethylacetamide (DMA)46 and caprolactam (CPL)47 containing lithium chloride were privileged solvents for HMF production because of the formation of DMA·Li+ or CPL·Li+ macrocations resulted in a high concentration of weakly ion-paired halide ions,46 which could promote the dehydration of fructose. However, these solvent systems have not been applicated into the heterogeneously catalytic fructose dehydration.
As discussed above, there have been many catalysts and solvents for the dehydration of fructose to produce HMF. Consideration of catalysts and solvents as a whole is the key and interesting point to obtain high HMF selectivity and yield from the dehydration of fructose. More efforts should be paid on this aspect. In this work, we synthesized a Nb-containing catalyst by the reaction of niobium chloride (NbCl5) and nitrilotris(methylenephosphonic acid) (NTMPA), denoted as Nb–NTMPA (Scheme 1). The as-prepared Nb–NTMPA could be used as an efficient heterogeneous catalyst for the dehydration of fructose to produce HMF in DMA–salts mixtures. It was found that the niobium phytic was very active and stable for the dehydration of fructose with a maximum yield of 85.6% using DMA–NaBr mixture as the reaction solvent. Meanwhile, we also discovered that the type of salts had significant impact on the activity of the reaction systems.
Results and discussion
Catalyst characterization
The synthetic procedure for Nb–NTMPA was presented in detail in the Experimental section. The prepared Nb–NTMPA was firstly examined by X-ray diffraction (XRD) method. The XRD pattern showed one broad diffraction peak, indicating that the obtained Nb–NTMPA was amorphous (Fig. 1a). The scanning electron microscopy (SEM, Fig. 1b) and transmission electron microscopy (TEM, Fig. 1c) demonstrated that the Nb–NTMPA was featureless. Meanwhile, N2 adsorption–desorption examination indicated that the Nb–NTMPA prepared was porous (Fig. 1d), and the average pore diameter, the Brunauer–Emmett–Teller (BET) surface area, and the pore volume calculated from the N2 adsorption–desorption were 5.5 nm, 25.2 m2 g−1, and 0.14 cm3 g−1, respectively. As shown in Fig. 1d, the N2 adsorption–desorption isotherm showed the type IV characteristic of mesoporous materials.48,49 The hysteresis loop was an intermediate between typical H1 and H2-type in the P/P0 range from 0.4 to 0.9, suggesting large uniform mesopores with a cage like pore structure connected by windows with a small size,49,50 which was characteristic of mesoporous materials according to IUPAC classification.39,48 Additionally, the pore size distribution indicated the existence of large mesopores in the prepared Nb–NTMPA (Fig. 1e). | ||
| Fig. 1 Characterization of the synthesized Nb–NTMPA. Powder XRD pattern (a), SEM image (b), TEM image (c), N2 adsorption–desorption isotherm (d), and the pore size distribution (e). | ||
Furthermore, X-ray photoelectron spectroscopy (XPS) showed the characteristic peaks of Nb5+ (207.4 eV for Nb 3d5/2 and 210.2 eV for Nb 3d3/2, Fig. 2a) and P5+ (133.6 eV for P 2p, Fig. 2b), suggesting the presence of Nb5+ and P5+ in the prepared catalyst. In addition, Fourier transform-infrared (FT-IR) technique showed one strong band at 1017 cm−1, suggesting the formation of Nb–O–P networks in the synthesized catalyst (Fig. 2c).39 Meanwhile, an additional band was also found at 615 cm−1, which was assigned to Nb–O stretching modes.39
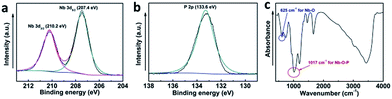 | ||
| Fig. 2 XPS spectra of Nb 3d (a), XPS spectra of P 2p (b), and FT-IR spectra of the prepared Nb–NTMPA (c). | ||
Effect of various salts on the dehydration activity
The effect of various salts on the dehydration of fructose was firstly examined over Nb–NTMPA at 100 °C with a reaction time of 1.5 h, and the results were given in Table 1. It could be seen that the yield of HMF was very low in the absence of salts (entry 1, Table 1). When salts with halide ion were added, the activity of the catalytic system was increased dramatically (entries 2–7, Table 1). When the anion of the salts was Cl−, the activity order of the salts was found to be LiCl > KCl > NaCl (entries 2, 4 and 6, Table 1), which was consistent with the order of the solubility of these salts in DMA. The higher solubility could be beneficial for the formation of DMA·M+ (M = Li, Na and K) macrocations and weakly ion-paired chloride ions, and thus promoted the dehydration of fructose. When the anion of the salts was changed to Br−, the difference of the activity was not noticeable (entries 3, 5 and 7, Table 1). Meanwhile, we found that the salts with Br− ion had better performance than the salts with Cl− ion when they had the same cation. This may be resulted from that Br− had weaker binding force with the cation than Cl−, which had been proved that weakly ion-paired halide ions favored the reaction. In addition, the salts with NO3− and SO42− did not show positive effect, which further indicated that the halide ions could promote the dehydration of fructose by the formation of hydrogen bonds between halide ions and the hydroxyl groups in fructose.46 Based on the results shown in Table 1, we chose NaBr for further study on the activity of Nb–NTMPA. In addition, we observed the formation of humin in our reaction system, which were likely generated through self-polymerization of HMF or cross-polymerization between fructose and HMF.51–53 Meanwhile, small amount of levulinic acid and formic acid could also be detected, which were generated from the rehydration of the formed HMF.| Entry | Solvent | Conversion (%) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 0.1 g fructose, 50 mg Nb–NTMPA, 2 g DMA, salts 0.2 g, reaction time 1.5 h, reaction temperature 100 °C.b Yields were determined by HPLC. | |||
| 1 | DMA | 6.1 | 3.6 |
| 2 | DMA + LiCl | 96.4 | 53.6 |
| 3 | DMA + LiBr | 100 | 81.9 |
| 4 | DMA + NaCl | 39.7 | 29.9 |
| 5 | DMA + NaBr | 100 | 85.6 |
| 6 | DMA + KCl | 47.7 | 39.4 |
| 7 | DMA + KBr | 98.6 | 80.5 |
| 8 | DMA + KNO3 | 7.8 | 4.3 |
| 9 | DMA + K2SO4 | 6.3 | 3.4 |
| 10 | DMA + NaNO3 | 5.9 | 4.8 |
| 11 | DMA + Na2SO4 | 4.2 | 2.7 |
Activity of different Nb-containing catalysts in DMA–NaBr mixtures
The performance of various Nb-containing catalysts for the dehydration of fructose in DMA–NaBr mixtures was studied (Table 2). The yield of HMF was very low in the absence of any catalysts (entry 1, Table 2). According to the experimental data, the as-prepared Nb–NTMPA (entry 4, Table 1) had similar catalytic activity with the homogeneous NbCl5 (entry 2, Table 1) and better performance than Nb2O5 (entry 3, Table 1).| Entry | Catalyst | Conversion (%) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 0.1 g fructose, 50 mg catalyst, 2 g DMA, NaBr 0.2 g, reaction time 1.5 h, reaction temperature 100 °C.b Yields were determined by HPLC. | |||
| 1 | None | 3.6 | 1.9 |
| 2 | NbCl5 | 100 | 80.5 |
| 3 | Nb2O5 | 97.3 | 73.4 |
| 4 | Nb–NTMPA | 100 | 85.6 |
| 5 | Nb–NTMPA (2nd) | 98 | 82.3 |
| 6 | Nb–NTMPA (3rd) | 95 | 80.2 |
There were two reasons for the higher activity of Nb–NTMPA than Nb2O5, including higher acidity and lower crystallinity. Firstly, the acidity of the prepared Nb–NTMPA and Nb2O5 was examined by NH3-TPD method. The results in Fig. 3 suggested that the acidity of Nb–NTMPA (0.22 mmol g−1) was higher than Nb2O5 (0.03 mmol g−1), which were calculated based on the desorbed NH3 (Fig. 3) at the temperatures from 100 °C to 350 °C. Further, the acid strength and amounts of Nb–NTMPA and Nb2O5 were determined by the n-butylamine titration method accurately,36 and the results in Table S1† indicated the higher acidity of Nb–NTMPA than Nb2O5, which was consistent with the NH3-TPD examination. In addition, the pyridine absorption FT-IR analysis indicated that there existed both Lewis (193 μmol g−1) and Brønsted acid (21 μmol g−1) sites in Nb–NTMPA (Fig. S1†). The Brønsted acid sites were resulted from the small amount of non-coordinated P–OH species of the phosphate groups of NTMPA, which also promoted the dehydration of fructose. In contrast, there were only Lewis acid sites (64 μmol g−1) existed in Nb2O5, which was less than those in Nb–NTMPA according to the results shown in Fig. S1.† The higher activity of Nb–NTMPA was beneficial for the dehydration of fructose into HMF with a higher reaction rate, which could decrease the formation of humin from the polymerization of HMF with fructose, and thus provided a higher selectivity of HMF than Nb2O5. Secondly, the prepared Nb–NTMPA had lower crystallinity than the used Nb2O5 (Fig. S2†), which was favorable for fructose to be in contact with the activity center of the Nb–NTMPA, and thus promote the dehydration of fructose with a higher activity. These two reasons resulted in that Nb–NTMPA had better activity than Nb2O5 at the same reaction conditions.
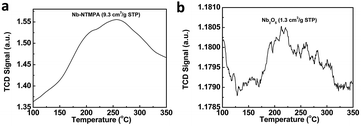 | ||
| Fig. 3 NH3-TPD spectra of Nb–NTMPA (a) and Nb2O5 (b). Due to the decomposition temperature of Nb–NTMPA was about 350 °C obtained from the thermogravimetric analysis (Fig. S3†). Therefore, we conducted NH3-TPD examination from 100 °C to 350 °C. The acidity of Nb–NTMPA and Nb2O5 can be calculated based on the desorbed NH3. | ||
The reusability of Nb–NTMPA was examined at the optimized reaction conditions (entries 4–7, Table 2). It was obvious that Nb–NTMPA suffered a minor decrease in activity within 3 cycles, which was probably caused by the loss of Nb–NTMPA when recovered from reaction systems and the active center occupied by humin adsorbed on the catalyst. We also examined the content of niobium in the reaction mixtures after the catalyst was removed and ca. 77 ppm of niobium (about 1.12 atom% Nb) was detected after the third run. Thus, we thought that partial leaching of niobium segments from the bulk catalyst phase could also reduce the activity of the catalyst. The Nb–NTMPA recovered after reusing three times was characterized by FT-IR (Fig. 4a), XRD (Fig. 4b), SEM (Fig. 4c), and TEM (Fig. 4d). It can be seen that the properties of Nb–NTMPA were not changed notably after being used. Additionally, N2 adsorption–desorption (Table S2†) and XPS (Table S3 and Fig. S4†) examinations proved that the surface characteristics and textural properties of fresh and three-times used Nb–NTMPA remained almost unchanged.
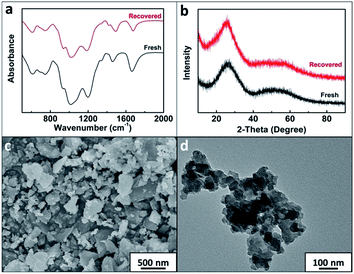 | ||
| Fig. 4 The characterization of the recovered Nb–NTMPA after reused three times. FT-IR spectrum (a), XRD pattern (b), SEM image (c), and TEM image (d). | ||
Influence of the salts amounts
Based on the results shown in Table 1, salts played a crucial role for the dehydration of fructose. Further study indicated that the amount of salts could also affect the yield of HMF. As shown in Fig. 5, the yield of HMF increased to a maximum of 85.6% with an increase in the amount of NaBr from 0 to 0.2 g which was probably caused by the increase of DMA·Na+ macrocations and weakly ion-paired bromide ions in the reaction system. However, the HMF yield kept almost constant with further increase in the amount of NaBr from 0.2 to 1 g, hinting that 0.2 g was the optimal NaBr amount for the reaction at our reaction conditions.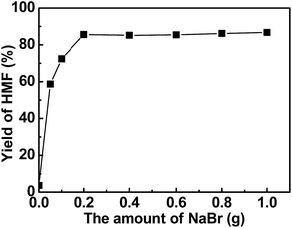 | ||
| Fig. 5 Influence of NaBr amounts. Reaction conditions: 0.1 g fructose, 50 mg Nb–NTMPA, 2 g DMA, reaction time 1.5 h, reaction temperature 100 °C. | ||
Effect of reaction temperatures and reaction time on the dehydration reaction
Fig. 6 shows the influence of reaction temperature on the dehydration of fructose to produce HMF in DMA–NaBr mixtures catalyzed by Nb–NTMPA at 60 °C, 80 °C and 100 °C. As shown in Fig. 6, we could find that the dehydration reaction was accelerated by increasing temperature, which was favorable to achieving higher yield in a shorter reaction time. When the reaction was conducted at 100 °C, the yield increased dramatically with time at the beginning, and reached the maximum (85.6%) at 1.5 h with a fructose conversion of 100%. Then, the yield decreased slightly with prolonged time, which may be due to the rehydration of the HMF produced.Conclusions
In summary, a novel Nb–containing catalyst, Nb–NTMPA, has been prepared and used as the heterogeneous catalyst for the dehydration of fructose to synthesize HMF using DMA–salts mixtures as the reaction solvents. High yield of 85.6% could be obtained from fructose in DMA–NaBr mixture at 100 °C with a reaction time of 1.5 h. The type of salts could affect the activity of the reaction systems and NaBr showed the best performance, which resulted from the formation of DMA·M+ macrocations and weakly ion-paired halide ions. We believe that the simple and efficient catalytic system has potential application for producing HMF from dehydration of carbohydrates.Experimental
Materials
Fructose (99%), sucrose (99%) and LiCl (99%) were purchased from Alfa Aesar. Inulin (95%), Nb2O5 (99.5%), niobium(V) chloride (99+%) and LiBr (99+%) were provided by J&K Scientific Ltd. N,N-Dimethylformamide (DMF), N,N-dimethylacetamide (DMA), NaBr, NaCl, KBr, KCl, KNO3, NaNO3, Na2SO4 and K2SO4 were A. R. grade and were obtained from Sinopharm Chemical Reagent Beijing Co., Ltd. Nitrilotris(methylenephosphonic acid) (ca. 2.2 mol L−1) was purchased from TCI (Shanghai) Development Co., Ltd. All chemicals were used as received.Synthesis of Nb–NTMPA
Niobium chloride (12 mmol) and nitrilotris(methylenephosphonic acid) (NTMPA, 10 mmol) were dissolved in DMF (600 mL) in a flask of 1000 mL. Then, triethylamine (120 mmol) was added into the solution dropwisely with a time of 1 h. After that, the mixture was firstly stirred for 10 h at room temperature and then aged under static conditions at 60 °C for 3 h. Finally, the slight yellow precipitate was separated by filtration, thoroughly washed with DMF, ethanol and ethyl ether. Before the characterization and utilization, the sample was washed by ethanol at 90 °C through Soxhlet extraction for 72 h to remove DMF completely. After that, the niobium phytic was dried at 80 °C under vacuum for 12 h.Catalyst characterization
FT-IR spectra were recorded on Bruker Tensor 27 IR spectrometer and the sample was prepared by the KBr pellet method. The BET surface area measurement and pore analysis were carried out by N2 adsorption at 77 K with Micromeritics ASAP 2020 V3.00 H (USA) surface area analyser. X-ray diffraction (XRD) measurements were conducted on an X-ray diffractometer (D/MAX-RC, Japan) operated at 40 kV and 200 mA with Cu Kα (λ = 0.154 nm) radiation. XPS measurements were carried out on an ESCAL Lab 220i-XL spectrometer at a pressure of ∼3 × 10−9 mbar (1 mbar = 100 Pa) using Al Kα as the excitation source (hν = 1486.6 eV) and operated at 15 kV and 20 mA. The scanning electron microscopy (SEM) measurements were performed on a Hitachi S-4800 Scanning Electron Microscope operated at 15 kV. The samples were spray-coated with a thin layer of platinum before observation. The transmission electron microscopy (TEM) images were obtained using a TEM JeoL-1011 with an accelerating voltage of 120 kV. The sample was dispersed in ethanol with the aid of sonication and dropped on an amorphous carbon film, supported on a copper grid, for the TEM analysis. Temperature-programmed desorption of ammonia (NH3-TPD) was performed on Micromeritics' AutoChem 2950 HP Chemisorption Analyzer. The catalysts (0.1475 g) were charged into the quartz reactor, and the temperature was increased from room temperature to 150 °C at a rate of 10 °C min−1 under a flow of He (50 cm3 min−1), and then the catalyst was kept at 150 °C for 4 h. After that, the temperature was decreased to 50 °C. NH3/He (10/90, 50 cm3 min−1) was pulsed into the reactor at 50 °C under a flow of He (10 cm3 min−1) until the acid sites were saturated with NH3. The adsorbed NH3 was removed by a flow of He (50 cm3 min−1). When the baseline was stable, the temperature was increased from 50 °C to 350 °C at a rate of 10 °C min−1.Conversion of carbohydrates into HMF
In a typical experiment, known amounts of carbohydrates, catalyst and salts were added in DMA (2 g) in a flask of 10 mL sealed with a glass stopper. The mixture was stirred at a fixed temperature for desired time. Then the mixture was cooled to room temperature immediately. The samples were analyzed by HPLC to obtain the yields. The yield of HMF was calculated by the following equation:Analysis methods for fructose conversion and HMF yield
The amount of HMF was analyzed by HPLC with Shimadzu LC-15C pump, Shimadzu UV-Vis SPD-15C detector at 282.0 nm, and a Supelcosil LC-18 5ìm column at 35 °C. Before analyzed, the reaction mixture was diluted to 1000 mL. Methanol/water solution (50/50 v/v) was used as the mobile phase at a flow rate of 0.8 mL min−1. The conversion of fructose was analyzed by HPLC with Shimadzu LC-20AT pump and Shimadzu RID-10A detector with a Benson BP-800 H+ carbohydrate column at 50 °C. Before analyzed, the reaction mixture was diluted to 500 mL. 5 mM H2SO4 aqueous solution was used as the mobile phase at flow rate of 0.6 mL min−1.Reusability of Nb–NTMPA
In the experiments to test the reusability of Nb–NTMPA, the catalyst was recovered by centrifugation, washed using DMA, ethanol and ethyl ether. After drying under vacuum at 80 °C for 12 h, the catalyst was reused for the next run.Acknowledgements
The authors thank National Natural Science Foundation of China (21503016, 21473252) for financial support.Notes and references
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Chem. Soc. Rev., 2012, 41, 8075–8098 RSC.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- R. A. Sheldon, Green Chem., 2014, 16, 950–963 RSC.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 CrossRef CAS PubMed.
- C. Li, X. Zhao, A. Wang, G. W. Huber and T. Zhang, Chem. Rev., 2015, 115, 11559–11624 CrossRef CAS PubMed.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS PubMed.
- M. Dashtban, A. Gilbert and P. Fatehi, RSC Adv., 2014, 4, 2037–2050 RSC.
- T. D. Swift, H. Nguyen, Z. Erdman, J. S. Kruger, V. Nikolakis and D. G. Vlachos, J. Catal., 2016, 333, 149–161 CrossRef CAS.
- R. Otomo, T. Yokoi and T. Tatsumi, ChemCatChem, 2015, 7, 4180–4187 CrossRef CAS.
- T. A. Werpy and G. Petersen, Top value added chemicals from biomass, U.S. Department of Energy (DOE), Golden, CO, DOE/GO-102004–1992, 2004 Search PubMed.
- R.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- J. Chen, J. Zhong, Y. Guo and L. Chen, RSC Adv., 2015, 5, 5933–5940 RSC.
- X. Kong, R. Zheng, Y. Zhu, G. Ding, Y. Zhu and Y.-W. Li, Green Chem., 2015, 17, 2504–2514 RSC.
- J. Lan, J. Lin, Z. Chen and G. Yin, ACS Catal., 2015, 5, 2035–2041 CrossRef CAS.
- Z. Huang, Y. Pan, Y. Chao, W. Shen, C. Wang and H. Xu, RSC Adv., 2014, 4, 13434–13437 RSC.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- Y. Shen, J. Sun, Y. Yi, B. Wang, F. Xu and R. Sun, J. Mol. Catal. A: Chem., 2014, 394, 114–120 CrossRef CAS.
- S. Siankevich, Z. Fei, R. Scopelliti, G. Laurenczy, S. Katsyuba, N. Yan and P. J. Dyson, ChemSusChem, 2014, 7, 1647–1654 CrossRef CAS PubMed.
- A. H. Jadhav, H. Kim and I. Taek-Hwang, Catal. Commun., 2012, 21, 96–103 CrossRef CAS.
- J. Zhang, X. Yu, F. Zou, Y. Zhong, N. Du and X. Huang, ACS Sustainable Chem. Eng., 2015, 3, 3338–3345 CrossRef CAS.
- Y. Ma, S. Qing, L. Wang, N. Islam, S. Guan, Z. Gao, X. Mamat, H. Li, W. Eli and T. Wang, RSC Adv., 2015, 5, 47377–47383 RSC.
- G. Sampath and S. Kannan, Catal. Commun., 2013, 37, 41–44 CrossRef CAS.
- F. H. Richter, K. Pupovac, R. Palkovits and F. Schüth, ACS Catal., 2013, 3, 123–127 CrossRef CAS.
- E. Nikolla, Y. Román-Leshkov, M. Moliner and M. E. Davis, ACS Catal., 2011, 1, 408–410 CrossRef CAS.
- H. B. Xie, Z. K. Zhao and Q. Wang, ChemSusChem, 2012, 5, 901–905 CrossRef CAS PubMed.
- N. Wang, Y. Yao, W. Li, Y. Yang, Z. Song, W. Liu, H. Wang, X.-F. Xia and H. Gao, RSC Adv., 2014, 4, 57164–57172 RSC.
- P. Carniti, A. Gervasini, S. Biella and A. Auroux, Chem. Mater., 2005, 17, 6128–6136 CrossRef CAS.
- X. H. Qi, H. X. Guo, L. Y. Li and R. L. Smith, ChemSusChem, 2012, 5, 2215–2220 CrossRef CAS PubMed.
- J. Zhao, C. Zhou, C. He, Y. Dai, X. Jia and Y. Yang, Catal. Today, 2016, 264, 123–130 CrossRef CAS.
- H. Wang, Q. Kong, Y. Wang, T. Deng, C. Chen, X. Hou and Y. Zhu, ChemCatChem, 2014, 6, 728–732 CrossRef CAS.
- D. A. Kotadia and S. S. Soni, Catal. Sci. Technol., 2013, 3, 469–474 CAS.
- X.-L. Shi, M. Zhang, Y. Li and W. Zhang, Green Chem., 2013, 15, 3438–3445 RSC.
- H. Xu, Z. Miao, H. Zhao, J. Yang, J. Zhao, H. Song, N. Liang and L. Chou, Fuel, 2015, 145, 234–240 CrossRef CAS.
- J. Chen, K. Li, L. Chen, R. Liu, X. Huang and D. Ye, Green Chem., 2014, 16, 2490–2499 RSC.
- Z.-Z. Yang, J. Deng, T. Pan, Q.-X. Guo and Y. Fu, Green Chem., 2012, 14, 2986–2989 RSC.
- F. Wang, H.-Z. Wu, C.-L. Liu, R.-Z. Yang and W.-S. Dong, Carbohydr. Res., 2013, 368, 78–83 CrossRef CAS PubMed.
- P. Carniti, A. Gervasini, S. Biella and A. Auroux, Catal. Today, 2006, 118, 373–378 CrossRef CAS.
- E. L. S. Ngee, Y. Gao, X. Chen, T. M. Lee, Z. Hu, D. Zhao and N. Yan, Ind. Eng. Chem. Res., 2014, 53, 14225–14233 CrossRef CAS.
- Y. Zhang, J. Wang, J. Ren, X. Liu, X. Li, Y. Xia, G. Lu and Y. Wang, Catal. Sci. Technol., 2012, 2, 2485–2491 CAS.
- J. Wang, W. Xu, J. Ren, X. Liu, G. Lu and Y. Wang, Green Chem., 2011, 13, 2678–2681 RSC.
- Z. Huang, W. Pan, H. Zhou, F. Qin, H. Xu and W. Shen, ChemSusChem, 2013, 6, 1063–1069 CrossRef CAS PubMed.
- T. Deng, X. Cui, Y. Qi, Y. Wang, X. Hou and Y. Zhu, Chem. Commun., 2012, 48, 5494–5496 RSC.
- P. Wrigstedt, J. Keskiväli and T. Repo, RSC Adv., 2016, 6, 18973–18979 RSC.
- C. Shi, J. Xin, X. Liu, X. Lu and S. Zhang, ACS Sustainable Chem. Eng., 2016, 4, 557–563 CrossRef CAS.
- K. V. Wagh, K. C. Badgujar, N. M. Patil and B. M. Bhanage, Curr. Org. Chem., 2016, 20, 736–751 CrossRef CAS.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- T. Chen and L. Lin, Chin. J. Chem., 2010, 28, 1773–1776 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- S. K. Das, M. K. Bhunia, A. K. Sinha and A. Bhaumik, ACS Catal., 2011, 1, 493–501 CrossRef CAS.
- S. K. Das, M. K. Bhunia and A. Bhaumik, Dalton Trans., 2010, 39, 4382–4390 RSC.
- S. K. R. Patil and C. R. F. Lund, Energy Fuels, 2011, 25, 4745–4755 CrossRef CAS.
- I. van Zandvoort, Y. Wang, C. B. Rasrendra, E. R. H. van Eck, P. C. A. Bruijnincx, H. J. Heeres and B. M. Weckhuysen, ChemSusChem, 2013, 6, 1745–1758 CrossRef CAS PubMed.
- Z. Zhang, Q. Wang, H. Xie, W. Lu and Z. K. Zhao, ChemSusChem, 2011, 4, 131–138 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra09664f |
| This journal is © The Royal Society of Chemistry 2016 |



