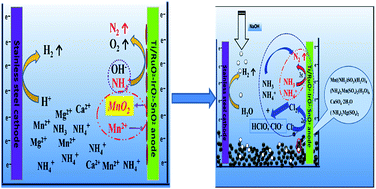
Mn2+ oxidation, playing counteractive role in electro-oxidation of ammonia, hinders the generation of free chlorine
Abstract
Ammonia in electrolytic manganese residue (EMR) constitutes potentially severe environmental risks. As an efficient, environment-friendly technology, electro-oxidation of ammonia in EMR was investigated in the present work. The influence of different metal ions coexisting with ammonia in EMR on the electro-oxidation of ammonia was evaluated and the relevant mechanism was deduced by phase transformation analysis through X-ray diffraction, X-ray fluorescence, scanning electron microscopy, flame atomic absorption spectrometry, and spectrophotometry. Results showed that (1) Mn2+, Ca2+, Mg2+, Na+, and K+ coexist with ammonia in the water-leach liquor of EMR, (2) Mn2+ inhibits the reaction whereas Ca2+ and Mg2+ demonstrate no distinct influence on the electro-oxidation of ammonia, and (3) Mn2+ and Cl− react emulously on the anode. MnO2 hinders the generation of free chlorine; thus, the presence of Mn2+ exerts a crucial impact on ammonia removal. So, it can be considered that further utilization of EMR can be realized by recycling Mn2+ first and then removing effectively ammonia through electro-oxidation method.

 Please wait while we load your content...
Please wait while we load your content...