Bio-inspired synthesis of hybrid tube-like structures based on CaCO3 and type I-collagen
Abstract
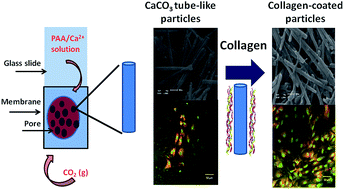
Although the exciting features exhibited by tube-like structures make them potential candidates for biomedical applications, few studies have dealt with the combination of tubular geometries and the biological responses of biominerals. This study reports on the synthesis of tube-like hybrid structures based on calcium carbonate, a biocompatible mineral, and collagen, the most abundant protein in bone tissue. A biomimetic model aided the synthesis of uniform tubular structures with controlled shape and diameter in the range of 100, 200, and 400 nm. Immersion of the particles in simulated body fluid (SBF) at 37 °C for 48 h allowed us to evaluate the bioactivity of the structures. Infrared spectroscopy revealed that biological apatite emerged in the material, confirming its bioactivity. Zeta-potential measurements attested to the high colloidal stability of the nanoparticles in the presence and absence of collagen. Confocal laser microscopy images confirmed that osteoblastic cells cultured in the presence of collagen-modified CaCO3 tube-like nanoparticles proliferated. The 100 nm particles presented special behavior—they adsorbed a larger amount of collagen due to their higher surface area.

 Please wait while we load your content...
Please wait while we load your content...