In vitro imaging of β-cells using fluorescent cubic bicontinuous liquid crystalline nanoparticles
V. Miceliag,
V. Melib,
M. Blanchard-Descec,
T. Bsaibessc,
M. Pampaloneag,
P. G. Conaldia,
C. Caltagironeb,
M. Obiols-Rabasad,
J. Schmidte,
Y. Talmone,
A. Casu*f and
S. Murgia*b
aDepartment of Laboratory Medicine and Advanced Biotechnologies, IRCCS-ISMETT (Istituto Mediterraneo per iTrapianti e Terapie ad Alta Specializzazione), via Tricomi 5, 90127 Palermo, Italy
bDepartment of Chemical and Geological Science, University of Cagliari, CNBS, CSGI, s.s. 554 bivio per Sestu, 09042 Monserrato CA, Italy. E-mail: murgias@unica.it; Tel: +39 070 675 4453
cUniversity of Bordeaux, Institut des Sciences Moléculaires, (CNRS UMR 5255), 33 405 Talence, France
dDivision of Physical Chemistry, Department of Chemistry, Naturvetarvägen 14, SE-22362 Lund, Sweden
eDepartment of Chemical Engineering, Technion – Israel Institute of Technology, Haifa 3200003, Israel
fDiabetes Service, Department for the Study and Treatment of Abdominal Diseases and Transplants, IRCCS-ISMETT (Istituto Mediterraneo per iTrapianti e Terapie ad Alta Specializzazione), via Tricomi 5, 90127 Palermo, Italy. E-mail: acasu@ismett.edu
gRi.MED Foundation, via Bandiera, 90133 Palermo, Italy
First published on 23rd June 2016
Abstract
To evaluate the potential of monoolein-based cubic bicontinuous liquid crystalline nanoparticles (cubosomes) as diagnostic tools in diabetes and pancreatic β-cell transplantation, fluorescent cubosomes were formulated, entrapping within the lipid bilayer a newly synthesized, potent, hydrophobic dye. Cryo-TEM, SAXS, and DLS were performed to assess the morphological and structural aspects of this formulation. Rat pancreatic β-cells (INS-1E) readily took up the cubosome nanoparticles, as revealed by in vitro internalization tests, and flow-cytometric analyses confirmed the persistence of the fluorescence for up to 24 hours. The cytotoxicity of cubosomes at various concentrations, evaluated with a real-time analysis of proliferation, revealed that the proliferation curves relative to the less concentrated formulation (5 μM in terms of monoolein content) were comparable to the untreated cultures. INS-1E cell cycle and apoptosis supported such results. In conclusion, this formulation, with dye content in the nanomolar range, seems to be useful for β-cell imaging.
1. Introduction
Nanotechnology applied to biomedical science offers unique opportunities to revolutionize the treatment of numerous diseases by exploiting nanomedicines, probably the most relevant outcome belonging to the merging of these two research domains. Nanomedicines are carriers with size in the submicrometer range, developed with the aim of improving the biodistribution of systemically administered diagnostic or therapeutic agents, improving treatment efficacy, and reducing undesirable side effects.1,2 Nanomedicine can combine multiple modalities on a single carrier, allowing for deeper understanding of in vivo processes. Their ability to simultaneously deliver drugs and imaging probes has paved the way for the advent of a new generation of theranostics.3 So far several nanomedicine formulations, including liposomes and polymeric nanoparticles, have been approved for clinical use by the FDA,4 and many others are currently under investigation. Among them, lipid-based cubic bicontinuous liquid crystalline nanoparticles, known as cubosomes, represent an emerging platform for the co-delivery of drugs and imaging probes.5,6 Indeed, similarly to the bulk bicontinuous cubic phases to which they belong,7 cubosomes possess an inner nanostructure characterized by curved, triply periodic, non-intersecting lipid bilayers, where hydrophobic molecules of biomedical or pharmaceutical interest can be hosted.8–11 Remarkably, these bilayers are arranged in space to form two disconnected and continuous water channels. Cubosomes are often regarded as the non-lamellar counterpart of liposomes, though, per unit of volume, they offer a higher hydrophobic volume and a larger surface exposed to water.12 Furthermore, their surface can be decorated with targeting residues to actively direct these nanoparticles towards specific tissues.13–15Several recent papers have explored the potential of cubosomes in cancer treatments. However, other equally severe pathologies, such as diabetes, could take advantage of this kind of nanoparticle. Type 1 diabetes (T1D) is a metabolic disease caused by the autoimmune destruction of the pancreatic β-cells, located in the islets of Langerhans in the pancreas. β-Cells produce and secrete insulin, a peptide hormone implicated in the maintenance of normal blood glucose levels. Chronic elevation of glucose in the blood of T1D patients causes vessel and organ dysfunction, which worsen quality of life, and cause premature death. Despite the increasing incidence of T1D worldwide, the questions of early detection, monitoring of the mechanisms leading to β-cells death, and monitoring of the effect of β-cells replacement therapies (i.e., islet transplantation) are still open, principally because of the difficulty in quantifying the mass of β-cells. To date, no methods are routinely clinically applicable for in vivo imaging of these cells. The development and implementation of reliable and accurate technologies for in vivo islet imaging is one of the priorities set by scientific committees and societies in the field of diabetes and β-cell transplantation.16
A number of strategies for pancreatic β-cell imaging have been attempted in the last 10 years, applying different techniques such as magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission computed tomography (SPECT), fluorescence, and combinations of them. For the most part, MRI strategies use commercially available iron oxide nanoparticles added to islet preparations before transplantation, with follow-up MRI imaging to detect a signal for up to 6 months after transplantation. There is conflicting data in the literature on long-term imaging capacity, and toxicity to the islets.17–19 Recently, GLP-1 receptor agonists or antibody-functionalized have been prepared and tested in rats, mice, and non-human primates, with promising results.20–23 MRI contrast agents for Zn2+ sensing have also been tested in vitro and in vivo.24,25 Tracers specifically targeted to the β-cells were applied. The most used targets were the GLP-1 receptor and VMAT2 (vesicular monoamine oxidase transporter 2). Specific ligands to these targets were labelled with radioactive compounds for SPECT and PET imaging. These strategies have been tested in animals and in humans to detect insulinomas and transplanted islets.26–29 Fluorescent dyes have also been applied in vitro, ex vivo and in vivo in animal models quite recently. The most interesting are a fluorescent GLP-1R agonist tested in mice and pigs,30 and a fluorescent glibenclamide molecule.31 Even if these approaches are promising and seem to be effective in animal models, the only applications in humans have been in islet transplantation, with limitations in the long-term detection of islets, and in tumour detection. Lack of specificity and high signal-to-noise ratio still limit most of the other strategies.
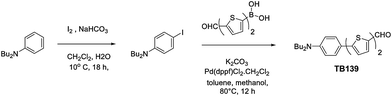
To assess the potential of cubosomes as an imaging tool for the evaluation of β-cells, we here report the in vitro interactions between β-cells and cubosomes loaded with a potent, hydrophobic fluorophore of innovative synthesis (Scheme 1, TB139).
2. Experimental section
2.1 Materials and methods
Monoolein (MO, 1-monooleoylglycerol, RYLO MG 19 PHARMA, glycerol monooleate, 98.1 wt%) was kindly provided by Danisco A/S, DK-7200, Grinsted, Denmark. Pluronic F108 (PEO132-PPO50-PEO132), N,N-dibutylaniline (97%), NaHCO3 (≥99.7%), K2CO3 (≥99%), Pd(dppf)Cl2, toluene (99.8%), methanol (≥99.8%), dichloromethane (99.8%), and petroleum ether (bp 40–60 °C) were purchased from Sigma-Aldrich. 5′-Formyl-2,2′-bithiophene-5-boronic acid was purchased from TCI. Distilled water passed through a Milli-Q water-purification system (Millipore) was used to prepare the samples.2.2 Synthesis of the fluorescent probe TB139
N,N-Dibutyl-4-iodoaniline obtained by iodination of commercially available N,N-dibutylanilinein the presence of NaHCO3 (ref. 32) (1.21 g, 3.65 mmol) and 5′-formyl-2,2′-bithiophene-5-boronic acid (0.95 g, 4.01 mmol) were dissolved in toluene (9 mL) and methanol (9 mL) in a dry round bottom flask flushed with argon. K2CO3 (1.25 g, 9.12 mmol) and Pd(dppf)Cl2 (148 mg, 0.18 mmol) were then added under argon, and the reaction mixture stirred at 80 °C for 6 hours. The mixture was filtered through a pad of celite, rinsed with dichloromethane, and the filtrate then concentrated under vacuum. The residue was further purified by silica-gel column chromatography using a 1/1 dichloromethane/petroleum ether mixture to yield 1 g (73%) of the desired compound as an orange solid. M.p. = 110–111 °C. Rf = 0.33 (petroleum ether/dichloromethane (1/1)). 1H NMR (300 MHz, CDCl3) δ 9.83 (s, 1H) 7.65 (d, J = 4 Hz, 1H), 7.45 (d, J = 8.9 Hz, 2H), 7.29 (d, J = 3.8 Hz, 1H), 7.20 (d, J = 4 Hz, 1H), 7.07 (d, J = 3.9 Hz, 1H), 6.63 (d, J = 8.9 Hz, 2H), 3.30 (t, J = 7.6 Hz, 4H), 1.59 (quint, J = 7.5 Hz, 4H), 1.37 (m, J = 7.4 Hz, 4H), 0.96 (t, J = 7.3 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 182.2, 148.1, 148.0, 147.8, 140.6, 137.5, 132.2, 127.3, 126.9, 123.1, 121.3, 120.3, 111.5, 50.7, 29.4, 20.3, 13.9. IR (KBr): 2955, 1661, 1606, 1456, 1231, 1052, 791 cm−1. HRMS (FD) calcd for C23H27NOS2 (M+) m/z 397.15341; found 397.15423. Anal. calcd for C23H27NOS2: C, 69.48; H, 6.84; N, 3.52. Found: C, 69.35; H, 7.00; N, 3.56.2.3 Cubosome preparation
Cubosomes were prepared by dispersing the appropriate amount of melted MO in a solution of Pluronic F108 using an ultrasonic processor UP100H by Dr Hielscher, cycle 0.9, amplitude 90%, for 10 minutes. To obtain fluorescent cubosomes, the fluorophore TB139 was dispersed in the melted monoolein with the help of an ultrasonic bath before dispersion in Pluronic F108. The sample volume was 4 mL with 96.4 wt% of water, 3.3 wt% of MO, and 0.3 wt% of Pluronic F108. The molar concentration of TB139 was 7.01 × 10−4.2.4 Dialysis and dye and drug-loading efficiency
TB139 is highly hydrophobic. In order to prove this property, before its encapsulation within cubosomes, solubility tests were carried out. TB139 was left under stirring in ultrapure water at room temperature overnight, at a nominal concentration equivalent to that present in cubosomes before dialysis (around 8 × 10−4 M). Absorbance and fluorescence spectra were acquired on the limpid solution obtained after centrifugation of the sample. A flat line was obtained in both cases, thus proving the virtually zero solubility in water of the dye. In the light of these experiments we assumed that a dialysis of 2 hours (sample/water of dialysis ratio was equal to 1/500) was largely sufficient for the elimination of the non-encapsulated dye. After loading with TB139, the cubosome dispersion was purified from the non-encapsulated dye by dialysis: 2 mL was loaded into a dialysis tubing cellulose membrane (14 kDa MW cutoff) and dialyzed against water (1000 mL) for 2 hours (by replacing the water after 1 hour) at 25 °C. The loading efficiency (E%), expressed as percentage of the amount of the dye present in the formulation before dialysis, was determined by UV-vis spectroscopy after disruption of cubosomes with methanol. TB139 content was quantified with a Thermo Nicolet Evolution 300 UV-VIS spectrophotometer at 449 nm, and an E% of 87.5 was registered.2.5 Photophysical characterization
The photophysical properties of fluorescent dye TB139 were analyzed with freshly prepared air equilibrated solutions at room temperature (20 °C) in spectroscopic grade chloroform. UV/Vis absorption spectra were recorded using a Jasco V-570 spectrophotometer. Steady-state fluorescence measurements were made on dilute solutions (optical density < 0.1) contained in standard 1 cm quartz cuvettes using a Horiba (FluoroLog or FluoroMax) spectrometer in photon-counting mode. Fully corrected emission spectra were obtained at λex = λmaxabs, with an optical density at λex ≤ 0.1 to minimize internal absorption. Fluorescence quantum yields were measured using cresyl violet in MeOH (Φf = 0.54) as a standard. Cubosome dispersions were diluted with Milli-Q water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) before making the photophysical measurements. The emission and excitation spectra were recorded with a Perkin Elmer LS 55 spectrofluorimeter.
300) before making the photophysical measurements. The emission and excitation spectra were recorded with a Perkin Elmer LS 55 spectrofluorimeter.
2.6 Cryogenic transmission electron microscopy (cryo-TEM)
Vitrified specimens were prepared in a controlled environment vitrification system (CEVS) at 25 °C and 100% relative humidity. A drop (∼3 μL) of the sample was placed on a perforated carbon film-coated copper grid, blotted with filter paper, and plunged into liquid ethane at its freezing point. The vitrified specimens were transferred to a Gatan 626 cryo-holder and observed at 120 kV acceleration voltage in an FEI Tecnai T12 G2 transmission electron microscope at about −175 °C in the low-dose imaging mode to minimize electron-beam radiation-damage. Images were digitally recorded with a Gatan US1000 high-resolution CCD camera.2.7 Small-angle X-ray scattering (SAXS)
The characterization of the nanoparticles' structure as a function of temperature was made with SAXS, using the Ganesha 300XL (SAXSLAB ApS, Skovlunde, Denmark). This instrument is equipped with a 2D 300 K Pilatus detector (Dectris Ltd., Baden, Switzerland) and a Genix 3D X-ray source (Xenocs SA, Sassenage, France), generating X-rays at a wavelength, λ, of 1.54 Å. The scattering data were collected at a q-range of 0.014 < q (Å−1) < 0.753, where the magnitude of the scattering vector, q, is defined as q = (4π/λ)sin(θ/2), and where θ is the scattering angle. The two-dimensional scattering pattern was radially averaged using SAXSGui software to obtain I(q). Measurements were made in 1.5 mm quartz capillaries (Hilgenberg GmbH, Malsfeld, Germany). The temperature was controlled by an external, re-circulating water bath within ±0.3 °C. The cubosome formulation was analysed by SAXS without further dilution or treatment. Each scattering measurements was performed for 2 hours after 1 hour of equilibration at the measurement temperature.Water channels radii of the reverse bi-continuous cubic phases were calculated using the relation rW = [(A0/−2πχ)1/2a] − L, where L is the length of the lipid hydrophobic chain (17 Å, in case of MO), a is the lattice parameter obtained from the SAXS analysis, and A0 and χ are the surface area and the Euler characteristic of the infinite periodic minimal surface geometries (Pn3m, A0 = 1.919, χ = −2; Im3m, A0 = 2.345, χ = −4). At least two Bragg peaks were used to estimate the errors associated with a and rW, unless otherwise indicated.
2.8 Light scattering
Dynamic light scattering (DLS) experiments were done to obtain the size evolution of the nanoparticles as a function of temperature. For this purpose we used a light scattering goniometer instrument (3D LS Spectrometer, LS Instruments, Fribourg, Switzerland), equipped with a 35 mW He–Ne laser light source (wavelength of 632.8 nm). The instrument implements the so-called cross-correlation scheme to suppress contributions from multiple scattering,33–35 together with a modulation unit.36 The samples were placed in 10 mm diameter cylindrical borosilicate disposable culture tubes (Fisherbrand, Thermo Fisher Scientific Inc., Waltham, USA), and kept at the measurement temperature in a temperature-controlled index-matching bath. Samples were equilibrated at the measurement temperature for 10 minutes before the experiment. The scattering angle was set at 90°, and each sample was measured at 10, 25, 37, and 50 °C five times for temperature. The initial decay rate, Γ, was derived from the second-order cumulant analysis of the normalized field autocorrelation function, which was used to calculate the apparent collective diffusion coefficient, D0, of the nanoparticles:| Γ = D0q2 | (1) |
2.9 Cell culture and treatment
The rat pancreatic β-cell line (INS-1E) was cultured in RPMI1640 medium with 11 mM glucose (Invitrogen, USA), supplemented with 10% fetal bovine serum (FBS, Hyclone, USA), 10 mmol L−1 HEPES, 1 mM sodium pyruvate, 2 mmol L−1 L-glutamine, 50 μM 2-mercaptoethanol, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin (Invitrogen, USA) at 37 °C and 5% CO2.Cubosomes loaded with TB139 fluorophore were added to the cell culture at the following concentrations: 5, 10, 20, and 100 μM of cubosomes (in terms of lipid content), at different incubation times, as detailed below.
2.10 Flow cytometric analysis
The internalization efficiency of cubosomes to the cells was investigated with flow cytometry (BD FACS ARIA II instrument, Becton Dickinson Biosciences). INS-1E cells grown at 40–50% confluence were incubated with or without (controls) TB139-labeled cubosomes (5–10–20–100 μM in terms of lipid content) for 1, 2, 4, and 24 hours, and analyzed for the evaluation of TB139 signal. INS-1E cells were detached with 0.05% trypsin–EDTA and washed twice with PBS before flow cytometry analysis. Non-specific fluorescence was evaluated on non-treated cells. The experiment was conducted in triplicate.2.11 Fluorescence microscopy
The cubosomes' internalization efficiency was also investigated with fluorescence microscopy observation in the same conditions described above. The TB139 signal was evaluated using EVOS™ fl Digital Inverted Fluorescence Microscope (Fisher Scientific, Paisley, Scotland, UK). Signal intensity was analyzed with ImageJ software (National Institute of health, USA).2.12 Assay of cell growth (xCELLigence)
Cell growth was measured in real time using xCELLigence technology (ACEA, San Diego, CA). The xCELLigence system was used according to manufacturers' instructions. Briefly, after background impedance determination, INS-1E cells (5 × 104 cells per well) were seeded into 16-well E-plates and incubated for 30 minutes at room temperature before installation in the Real-Time CellAnalyzer station in a conventional cell incubator at 37 °C and 5% CO2. The cells were grown for 48 hours before incubation with or without (control) 5, 10, 20, and 100 μM of cubosomes in the log growth phase. The time point 0 hours was determined when cells were exposed to the nanoparticles. The cells were treated with cubosomes for the duration of entire experiment. The impedance was measured every 15 minutes and expressed as cell index (CI), defined as (Rn − Rb)/15, where Rn is the impedance at a given time point, and Rb is the background impedance. Each culture condition was carried out in quadruplicate. The normalized CI was determined by the CI at a given time point divided by the CI at the normalization time point. Slopes of growth curves were determined in the first 24 hours after treatment.2.13 Cell cycle analysis
The cell cycle was measured by propidium iodide (PI) (Sigma, St. Louis, MO, USA) staining (50 μg mL−1). INS-1E cells 60–70% confluent were incubated with or without (controls) 5, 10, 20, and 100 μM of cubosomes for 24 hours. Cells were then fixed with 1 mL of 70% ethanol in PBS at −20 °C, and then washed once with PBS and subsequently re-suspended in 1 mL PI containing RNAse (250 μg mL−1) for 60 minutes at room temperature, and protected from light. PI fluorescence was detected at an excitation wavelength of 488 nm and an emission wavelength of 575 nm using a BD FACS ARIA II instrument, and was analysed with Diva 6.1.2 software (Becton Dickinson Biosciences). The experiment was conducted in triplicate.2.14 Apoptosis and necrosis analysis
Apoptosis/necrosis were analyzed with Annexin-V-FITC-PI assay kit following the manufacturer's staining protocols (Annexin-V-Fluos, Roche Applied Sciences, Germany). INS-1E cells 60–70% confluent were incubated with or without (controls) 5, 10, 20, and 100 μM of cubosomes for 24 hours. Annexin-V fluorescence was detected at an excitation wavelength of 488 nm and an emission wavelength of 525 nm, while PI fluorescence was detected at an excitation wavelength of 488 nm and an emission wavelength of 575 nm using a BD FACS ARIA II instrument. The experiment was conducted in triplicate and the data were analyzed with Diva 6.1.2 (Becton Dickinson Biosciences).2.15 Statistics
At least three independent experiments were done for each condition, and were expressed as the mean ± standard deviation (SD). Data from different groups were compared using the Student's t test. Differences between the groups were considered significant at a value of p < 0.05.3. Results and discussion
3.1 Physicochemical and photophysical characterization of fluorescent cubosomes
The lipophilic fluorescent probe TB139 was prepared via a multistep synthesis from the commercially available N,N-dibutylaniline (Scheme 1). First N,N-dibutyl-4-iodoaniline was obtained by iodination of N,N-dibutylanilinein the presence of NaHCO3, then subjected to a Suzuki–Miyaura cross-coupling with 5′-formyl-2,2′-bithiophen-5-boronic acid leading to TB139 in 73% yield. This dye is highly soluble in organic environments. It strongly absorbs in the UV and visible region, and emits in the visible region. The intense low-energy absorption band (λmaxabs = 451 nm, εmax = 3.1 × 104 M−1) is characteristic of an intramolecular charge transfer (ICT) transition. The dye is also a bright yellow-orange emitter in low to medium polarity solvents (λmaxem = 608 nm, Φf = 0.84 in CHCl3). Moreover, TB139 offers the advantage of a high quantum yield also after encapsulation inside the cubosomes (Φ = 0.39).A cryo-TEM37 image of the fluorescent cubosomes formulation is shown in Fig. 1. A number of quasi-spherical nanoparticles are discernible showing a dense, dark inner nanostructure (the lipid bilayers) intersected by a regular alternation of bright spots (the water channels). As often occurs in cubosome formulations, the presence of small unilamellar vesicles is also noticed.
To definitively assess the nanostructure of these nanoparticles, the formulation was further investigated with SAXS and DLS. Moreover, these physicochemical characterizations were made at both room and physiological temperature. Diffractograms acquired on cubosomes loaded with TB139 at 25 and 37 °C are shown in Fig. 2. At least four Bragg peaks were detected with relative positions √2:√3:2:√6, indexed as the (110), (111), (200), and (211) reflections of a double diamond inverse bicontinuous cubic phase (Pn3m crystallographic space group). However, a weak reflection at low q, indexed as the (110) reflection of a primitive inverse bicontinuous cubic phase (Im3m crystallographic space group), revealed that the cubosomes' nanostructure is, indeed, biphasic. The coexistence of the two bicontinuous cubic phases is well documented in the literature, and can be ascribed to the Pn3m to Im3m intercubic phase transition provoked by the Pluronic used to stabilize the formulation against coalescence when, after saturation of the surface of the cubosomes, this polymer starts to be incorporated with the monoolein, participating to the formation of the inner structure of the nanoparticles.38,39
 | ||
| Fig. 2 I(q) vs. q data obtained by SAXS experiments performed on the same cubosome formulation as in Fig. 1 at 25 and 37 °C. The Miller indices are reported above the corresponding Bragg peaks, with the indication of the space group. | ||
The lattice parameters, a, and the water channels radii, rW, calculated for both these bicontinuous cubic phases are reported in Table 1, and indicates that the nanostructure is basically not altered by the increase in temperature, though the structure slightly shrinks (a and rW decrease) because of the higher negative curvature at the lipid/water interface induced by the enhanced disorder of the MO chains at higher temperature.
DLS results confirmed that the size and the polydispersity of the nanoparticles are also unaffected by the temperature increase, with the formulation showing an average hydrodynamic radius of 80 nm, and a polydispersity index of 0.15 at both tested temperatures. These results are fully comparable to others previously reported by our group concerning empty cubosome formulations (not loaded with drugs and/or imaging probes).40,41 This fact confirms that the insertion of the TB139 fluorescent probe within the lipid matrix (at the concentration here used) neither altered the local MO dynamics nor its effective packing parameter,42 with the preservation of the original nanostructure.
The emission and excitation spectra of the cubosome formulations recorded after 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 dilution in ultrapure water are reported in Fig. 3. Excitation at 449 nm results in an emission at 600 nm (solid line), and the excitation spectra fixed at 600 nm (dashed line) is in perfect accordance with the absorption spectrum of the free dye in CHCl3 (see inset in Fig. 3 solid line). Due to the turbidity of the dispersion, the determination of the quantum yield of the dye in the nanoparticles was not feasible.
300 dilution in ultrapure water are reported in Fig. 3. Excitation at 449 nm results in an emission at 600 nm (solid line), and the excitation spectra fixed at 600 nm (dashed line) is in perfect accordance with the absorption spectrum of the free dye in CHCl3 (see inset in Fig. 3 solid line). Due to the turbidity of the dispersion, the determination of the quantum yield of the dye in the nanoparticles was not feasible.
 | ||
| Fig. 3 Normalized emission (dashed line, λexc = 449 nm) and excitation (solid line, λem = 600 nm) spectra of the aqueous cubosome formulation. | ||
3.2 Cell internalization
To evaluate the potential of this cubosome formulation as a diagnostic tool in diabetes, it is essential to examine their uptake in β-cells. For this purpose, a rat pancreatic β-cell line (INS-1E) was incubated without (controls) or with different concentrations of cubosomes. Different exposure times were chosen (1, 2, 4, and 24 hours) with the aim of mimicking exposure after a bolus administration or continuous infusion or re-circulation. The internalization efficiency was evaluated in parallel with imaging under microscope and flow cytometry quantification. After incubation, cubosome red fluorescence was distributed homogeneously inside the cells but outside of the nucleus.Fig. 4A shows some representative images of INS-1E cells after cubosome internalization. A progressive increase of the fluorescent signal was detected with time and concentration, and this was confirmed by both fluorescence intensity analysis (Fig. 4B) and flow-cytometric analysis (Fig. 4C) of the INS-1E cells incubated with cubosomes. With both methods, the fluorescence was detectable at all times, only after exposure of cells to 100 μM cubosome solution. At lower concentrations, the fluorescence was detectable after 2 hours exposure, increased after 4 hours and reached a plateau at 24 hours exposure.
At 5 μM concentration fluorescence was detectable only after 4 and 24 hours incubation. Flow-cytometric analyses showed the signal persistence up to 24 hours, and a reduction of mean fluorescence intensity after 72 hours, likely caused by the split of fluorescence into separate cell populations due to the cell duplication process (data not shown).
3.3 Assessment of cell cytotoxicity
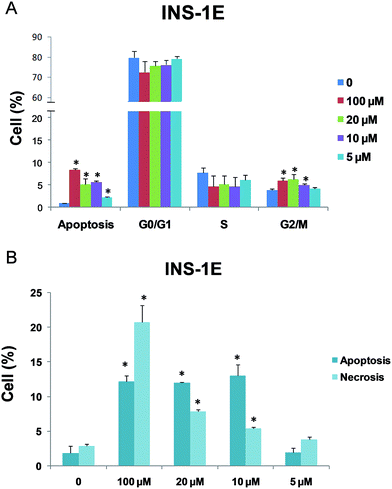
The potential cytotoxicity of monoolein-based cubosomes against various cell lines was recently explored in different papers by several authors, showing a certain degree of cellular toxicity induced by monoolein-based cubosomes. Such cytotoxicity varies depending on the formulation (monoolein/Pluronic ratio, type of Pluronic used) and, particularly, by the cell line investigated.40,43–47 Remarkably, haemolytic properties of this kind of nanoparticles were also reported,48 although the formulation can be adapted to minimize/avoid this drawback.49 Here, the toxic effect of the cubosomes formulation containing the TB139 fluorophore was assessed in a pancreatic β-cell line. The effect on cell growth after exposure to 5, 10, 20 or 100 μM of cubosome formulation was evaluated by means of a real time analysis of proliferation carried out with an xCELLigence system. Cell proliferation capacity was considered an indicator of cell wellbeing. Only the proliferation curves relative to the 5 μM NPs were comparable to the untreated cultures. In contrast, when compared with the control, significant reduction in the cell index (CI) values was observed for INS-1E cells treated with 10–20–100 μM cubosome formulation (Fig. 5A and B). Moreover, INS-1E cells underwent severe loss of morphology when exposed to 100 μM of cubosomes for 24 hours, while there was no change in morphology when treated with 5 μM of cubosomes. This indicates that cells exposed to the cubosomes at 5 μM concentration preserved their viability, spreading capacity, and structure over the observation period (Fig. 5C).Because an effect was seen on cell proliferation, a more in-depth analysis was done to identify which step of the cell cycle entry and progression was affected. As shown in Fig. 6A the number of cells blocked in the G2–M phase increased (5.9%, 6.2%, 5%) after 24 hours of 100, 20, and 10 μM cubosome treatment, respectively, compared with untreated cells (3.9%), while the 5 μM cubosome formulation did not show any effect on the number of blocked cells in G2–M (4.2%) as compared with untreated cells. No significant difference of cell populations in G0–G1 and S phase after 24 hours of treatment was seen at any of the concentrations applied (Fig. 6A). A significant reduction of apoptotic cellsupon exposure to the cubosome formulation at 5 μM (2.15%) compared to exposure to 100, 20, and 10 μM cubosome formulation (8.4%, 5.2% and 5.6% respectively) was seen. A more in depth analysis of apoptosis was then done with Annexin V/propidium iodide staining. Only 5 μM cubosome treatment did not significantly affect apoptosis/necrosis (2% and 3.85% respectively) if compared to control (1.85% and 2.95% respectively), while a significant apoptosis was induced by 100, 20, 10 μM cubosome treatment. Also a significant necrosis was induced by 100, 20, 10 μM cubosome treatment (20.7%, 7.9% and 5.4% respectively). Therefore, the cell cycle and apoptosis/necrosis analysis confirmed what was seen through the xCELLigence apparatus, with minimal or no effect of 5 μM concentration on cell wellbeing. The reduction of cell proliferation with higher cubosome concentrations seems related to an increased cell apoptosis/necrosis and block in the G2–M phase.
4. Conclusions
Fluorescent cubosome nanoparticles were formulated by embedding within the monoolein palisade a newly synthesized dye, TB139. The physicochemical analysis, conducted by cryo-TEM, SAXS, and DLS confirmed that TB139 can be incorporated in the nanoparticles without altering the original morphological and topological features of the monoolein-based cubosomes. The photophysical investigation demonstrated that encapsulation of TB139 within the cubosomes did not alter the fluorescent properties of the dye. Compared to other dyes suitable for biomedical applications that emit in the same spectral window (around 600 nm), TB139 offers the advantage of a high quantum yield both in organic solvents and inside the cubosomes. The cubosome formulation was then tested at different concentrations for the imaging of a β-cell line, showing that it is internalized by the cells, and is well tolerated when used at low concentrations. This result was achieved thanks to the powerful fluorescent dye chosen, which allowed the reduction of cubosome concentration to be applied to the cells, maintaining clear fluorescence detection with either microscope imaging or flow cytometry.Recently, various examples of commercially available or synthetically modified fluorescent dyes were proposed for the imaging of β-cells, both in vitro and in vivo (VT750, AF680, bodipy analogous, rhodamine derivatives, just to cite a few).30,31,50–54 The concentrations used were normally in the range 1–50 μM. However, in some cases the concentration of the dyes could be as low as nanomolar level, as in our case. Indeed, TB139 is 3.77 × 10−8 M in the 5 μM monoolein sample, although it allows the imaging of the β-cells still after 24 hours. Remarkably, although some examples of magnetic nanoparticles for MRI imaging have been reported in the literature,55,56 to the best of our knowledge, the formulation here proposed is the first example of nanoparticles developed so far for the fluorescent imaging of β-cells.57
Given their similarity to liposomes, cubosomes have always attracted great attention as possible nanocarriers for IV administration. However, papers devoted to this topic have mainly focused on the use of cubosomes in cancer treatments, although other uses of this kind of nanoparticles, e.g. for siRNA delivery, were reported.58,59 Here, we successfully evaluated the use of these cubic liquid crystalline nanoparticles as tools for the imaging of pancreatic β-cells, revealing their potential for future in vitro and in vivo applications in diabetes diagnosis and treatments.
Acknowledgements
S. M. and V. Meli acknowledge financial support from the European Commission under the Seventh Framework Program by means of the grant agreement for the Integrated Infrastructure Initiative N. 262348 European Soft Matter Infrastructure (ESMI). Financial support by MIUR (Project PRIN 2010BJ23MN_002), FondazioneBanco di Sardegna and RegioneAutonomadellaSardegna (CRP-59699) are gratefully acknowledged. SardegnaRicerche Scientific Park (Pula, CA, Italy) is acknowledged for free access to facilities of the Nanobiotechnology Laboratory. The cryo-TEM work was performed at the Technion Laboratory for Electron Microscopy of Soft Matter, supported by the Technion Russell Berrie Nanotechnology Institute (RBNI). The authors thank Dr A. Natalicchio (University of Bari, Italy) for kindly providing the INS-1E cells. M. B. D. gratefully acknowledges ConseilRégionald'Aquitaine for financial support (Chaired'Accueil grant). T. B. received a fellowship from the University of Bordeaux. Part of this study was conducted within the context of the Laboratory of Excellence TRAIL ANR-10-LABX-57.References
- T. Lammers, S. Aime, W. E. Hennink, G. Storm and F. Kiessling, Acc. Chem. Res., 2011, 44, 1029–1038 CrossRef CAS PubMed.
- O. Veiseh, J. W. Gunn and M. Zhang, Adv. Drug Delivery Rev., 2010, 62, 284–304 CrossRef CAS PubMed.
- J. A. Barreto, W. O'Malley, M. Kubeil, B. Graham, H. Stephan and L. Spiccia, Adv. Mater., 2011, 23, H18–H40 CrossRef CAS PubMed.
- A. Z. Wang, R. Langer and O. C. Farokhzad, Annu. Rev. Med., 2012, 63, 185198 CrossRef PubMed.
- I. D. Azmi, S. M. Moghimi and A. Yaghmur, Ther. Delivery, 2015, 6, 1347–1364 CrossRef CAS PubMed.
- X. Mulet, B. J. Boyd and C. J. Drummond, J. Colloid Interface Sci., 2013, 393, 1–20 CrossRef CAS PubMed.
- M. Monduzzi, S. Lampis, S. Murgia and A. Salis, Adv. Colloid Interface Sci., 2014, 205, 48–67 CrossRef CAS PubMed.
- S. T. Hyde, Curr. Opin. Solid State Mater. Sci., 1996, 1, 653–662 CrossRef CAS.
- S. Hyde, B. W. Ninham, S. Andersson, K. Larsson, T. Landh, Z. Blum and S. Lidin, The Language of Shape, Elsevier, 1997 Search PubMed.
- K. Larsson, Nature, 1983, 304, 664 CrossRef.
- T. Landh, J. Phys. Chem., 1994, 98, 8453–8467 CrossRef CAS.
- V. Meli, C. Caltagirone, A. M. Falchi, S. T. Hyde, V. Lippolis, M. Monduzzi, M. Obiols-Rabasa, A. Rosa, J. Schmidt, Y. Talmon and S. Murgia, Langmuir, 2015, 31, 9566–9575 CrossRef CAS PubMed.
- J. Zhai, J. A. Scoble, N. Li, G. Lovrecz, L. J. Waddington, N. Tran, B. W. Muir, G. Coia, N. Kirby, C. J. Drummond and X. Mulet, Nanoscale, 2015, 7, 2905–2913 RSC.
- C. Caltagirone, A. M. Falchi, S. Lampis, V. Lippolis, V. Meli, M. Monduzzi, L. Prodi, J. Schmidt, M. Sgarzi, Y. Talmon, R. Bizzarri and S. Murgia, Langmuir, 2014, 30, 6228–6236 CrossRef CAS PubMed.
- S. Aleandri, D. Bandera, R. Mezzenga and E. M. Landau, Langmuir, 2015, 31, 12770–12776 CrossRef CAS PubMed.
- S. T. Bartlett, J. F. Markmann, P. Johnson, O. Korsgren, B. J. Hering, D. Scharp, T. W. H. Kay, J. Bromberg, J. S. Odorico, G. C. Weir, N. Bridges, R. Kandaswamy, P. Stock, P. Friend, M. Gotoh, D. K. C. Cooper, C.-G. Park, P. O'Connell, C. Stabler, S. Matsumoto, B. Ludwig, P. Choudhary, B. Kovatchev, M. R. Rickels, M. Sykes, K. Wood, K. Kraemer, A. Hwa, E. Stanley, C. Ricordi, M. Zimmerman, J. Greenstein, E. Montanya and T. Otonkoski, Report from IPITA-TTS Opinion Leaders Meeting on the Future of β-Cell Replacement, 2016, vol. 100 Search PubMed.
- S. Borot, L. A. Crowe, G. Parnaud, F. Ris, R. Meier, L. Giovannoni, Y. D. Müller, S. Lacotte, P. Morel, C. Toso, D. Bosco, J.-P. Vallee and T. Berney, Transplantation, 2013, 96, 438–444 CrossRef CAS PubMed.
- M. L. Malosio, A. Esposito, A. Poletti, S. Chiaretti, L. Piemonti, R. Melzi, R. Nano, F. Tedoldi, T. Canu, P. Santambrogio, C. Brigatti, F. De Cobelli, P. Maffi, A. Secchi and A. Del Maschio, Am. J. Transplant., 2009, 9, 2372–2382 CrossRef CAS PubMed.
- M. L. Malosio, A. Esposito, C. Brigatti, A. Palmisano, L. Piemonti, R. Nano, P. Maffi, F. De Cobelli, A. Del Maschio and A. Secchi, Cell Transplant., 2015, 24, 2285–2296 Search PubMed.
- D. Z. Balla, S. Gottschalk, G. Shajan, S. Ueberberg, S. Schneider, M. Hardtke-Wolenski, E. Jaeckel, V. Hoerr, C. Faber, K. Scheffler, R. Pohmann and J. Engelmann, Contrast Media Mol. Imaging, 2013, 8, 495–504 CrossRef CAS PubMed.
- P. Wang, C. Schuetz, P. Vallabhajosyula, Z. Medarova, A. Tena, L. Wei, K. Yamada, S. Deng, J. F. Markmann, D. H. Sachs and A. Moore, Transplantation, 2015, 99, 1574–1581 CrossRef CAS PubMed.
- L. Vinet, S. Lamprianou, A. Babič, N. Lange, F. Thorel, P. L. Herrera, X. Montet and P. Meda, Diabetologia, 2015, 58, 304–312 CrossRef CAS PubMed.
- B. Yang, H. Cai, W. Qin, B. Zhang, C. Zhai, B. Jiang and Y. Wu, Int. J. Nanomed., 2013, 8, 3977–3990 Search PubMed.
- A. J. M. Lubag, L. M. De Leon-rodriguez, S. C. Burgess and A. D. Sherry, PNAS, 2011, 108, 18400–18405 CrossRef CAS PubMed.
- G. J. Stasiuk, F. Minuzzi, M. Sae-Heng, C. Rivas, H. P. Juretschke, L. Piemonti, P. R. Allegrini, D. Laurent, A. R. Duckworth, A. Beeby, G. A. Rutter and N. J. Long, Chem.–Eur. J., 2015, 21, 5023–5033 CrossRef CAS PubMed.
- E. Christ, D. Wild, S. Ederer, M. Béhé, G. Nicolas, M. E. Caplin, M. Brändle, T. Clerici, S. Fischli, C. Stettler, P. J. Ell, J. Seufert, B. Gloor, A. Perren, J. C. Reubi and F. Forrer, Lancet Diabetes Endocrinol., 2013, 1, 115–122 CrossRef CAS PubMed.
- M. Brom, L. Joosten, C. Frielink, O. Boerman and M. Gotthardt, Diabetes, 2015, 64, 1324–1328 CrossRef CAS PubMed.
- A. Garcia, A. Venugopal, M.-L. Pan and J. Mukherjee, Diabetes Technol. Ther., 2014, 16, 640–643 CrossRef CAS PubMed.
- F. Pattou, J. Kerr-Conte and D. Wild, N. Engl. J. Med., 2010, 363, 1289–1290 CrossRef PubMed.
- T. Reiner, G. Thurber, J. Gaglia, C. Vinegoni, C. W. Liew, R. Upadhyay, R. H. Kohler, L. Li, R. N. Kulkarni, C. Benoist, D. Mathis and R. Weissleder, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 12815–12820 CrossRef CAS PubMed.
- A. Babič, S. Lamprianou, L. Vinet, N. Stransky-Heilkron, C. Xayaphoummine, M. A. Campo, H. Glombik, A. Schulte, H. P. Juretschke, X. Montet, P. Meda and N. Lange, Biomaterials, 2016, 75, 1–12 CrossRef PubMed.
- O. Mongin, T. R. Krishna, M. H. V. Werts, A.-M. Caminade, J.-P. Majoral and M. Blanchard-Desce, Chem. Commun., 2006, 915–917 RSC.
- G. D. J. Phillies, Phys. Rev. A At., Mol., Opt. Phys., 1981, 24, 1939–1943 CrossRef CAS.
- K. Schätzel, J. Mod. Opt., 1991, 38, 1849–1865 CrossRef.
- C. Urban and P. Schurtenberger, J. Colloid Interface Sci., 1998, 207, 150–158 CrossRef CAS PubMed.
- I. D. Block and F. Scheffold, Rev. Sci. Instrum., 2010, 81, 123107 CrossRef PubMed.
- Y. Talmon, J. Mol. Liq., 2015, 210, 2–8 CrossRef CAS.
- M. Nakano, A. Sugita, H. Matsuoka and T. Handa, Langmuir, 2001, 17, 3917–3922 CrossRef CAS.
- J. Gustafsson, H. Ljusberg-Wahren, M. Almgren and K. Larsson, Langmuir, 1997, 13, 6964–6971 CrossRef CAS.
- S. Murgia, A. M. Falchi, V. Meli, K. Schillén, V. Lippolis, M. Monduzzi, A. Rosa, J. Schmidt, Y. Talmon, R. Bizzarri and C. Caltagirone, Colloids Surf., B, 2015, 129, 87–94 CrossRef CAS PubMed.
- A. M. Falchi, A. Rosa, A. Atzeri, A. Incani, S. Lampis, V. Meli, C. Caltagirone and S. Murgia, Toxicol. Res., 2015, 4, 1025–1036 RSC.
- S. Murgia, F. Caboi and M. Monduzzi, Chem. Phys. Lipids, 2001, 110, 11–17 CrossRef CAS PubMed.
- B. W. Muir, D. P. Acharya, D. F. Kennedy, X. Mulet, R. A. Evans, S. M. Pereira, K. L. Wark, B. J. Boyd, T.-H. Nguyen, T. M. Hinton, L. J. Waddington, N. Kirby, D. K. Wright, H. X. Wang, G. F. Egan and B. A. Moffat, Biomaterials, 2012, 33, 2723–2733 CrossRef CAS PubMed.
- S. Murgia, A. M. Falchi, M. Mano, S. Lampis, R. Angius, A. M. Carnerup, J. Schmidt, G. Diaz, M. Giacca, Y. Talmon and M. Monduzzi, J. Phys. Chem. B, 2010, 114, 3518–3525 CrossRef CAS PubMed.
- S. Deshpande, E. Venugopal, S. Ramagiri, J. R. Bellare, G. Kumaraswamy and N. Singh, ACS Appl. Mater. Interfaces, 2014, 6, 17126–17133 Search PubMed.
- T. M. Hinton, F. Grusche, D. Acharya, R. Shukla, V. Bansal, L. J. Waddington, P. Monaghan and B. W. Muir, Toxicol. Res., 2014, 3, 11–22 RSC.
- N. Tran, X. Mulet, A. M. Hawley, T. M. Hinton, S. T. Mudie, B. W. Muir, E. C. Giakoumatos, L. J. Waddington, N. M. Kirby and C. J. Drummond, RSC Adv., 2015, 5, 26785–26795 RSC.
- J. Barauskas, C. Cervin, M. Jankunec, M. Špandyreva, K. Ribokaitė, F. Tiberg and M. Johnsson, Int. J. Pharm., 2010, 391, 284–291 CrossRef CAS PubMed.
- V. Jain, N. K. Swarnakar, P. R. Mishra, A. Verma, A. Kaul, A. K. Mishra and N. K. Jain, Biomaterials, 2012, 33, 7206–7220 CrossRef CAS PubMed.
- N. Y. Kang, S. C. Lee, S. J. Park, H. H. Ha, S. W. Yun, E. Kostromina, N. Gustavsson, Y. Ali, Y. Chandran, H. S. Chun, M. Bae, J. H. Ahn, W. Han, G. K. Radda and Y. T. Chang, Angew. Chem., Int. Ed., 2013, 52, 8557–8560 CrossRef CAS PubMed.
- S. M. Clardy, E. J. Keliher, J. F. Mohan, M. Sebas, C. Benoist, D. Mathis and R. Weissleder, Bioconjugate Chem., 2014, 25, 171–177 CrossRef CAS PubMed.
- J. Broichhagen, M. Schönberger, S. C. Cork, J. A. Frank, P. Marchetti, M. Bugliani, A. M. J. Shapiro, S. Trapp, G. A. Rutter, D. J. Hodson and D. Trauner, Nat. Commun., 2014, 5, 5116 CrossRef CAS PubMed.
- B. K. Agrawalla, Y. Chandran, W. H. Phue, S. C. Lee, Y. M. Jeong, S. Y. D. Wan, N. Y. Kang and Y. T. Chang, J. Am. Chem. Soc., 2015, 137, 5355–5362 CrossRef CAS PubMed.
- L. Zhang, T. Navaratna, J. Liao and G. M. Thurber, Bioconjugate Chem., 2015, 26, 329–337 CrossRef CAS PubMed.
- P. Wang, B. Yoo, J. Yang, X. Zhang, A. Ross, P. Pantazopoulos, G. Dai and A. Moore, Diabetes, 2014, 63, 1465–1474 CrossRef CAS PubMed.
- K. Oishi, Y. Miyamoto, H. Saito, K. Murase, K. Ono, M. Sawada, M. Watanabe, Y. Noguchi, T. Fujiwara, S. Hayashi and H. Noguchi, PLoS One, 2013, 8, 4–10 Search PubMed.
- M. J. Kim and D. Y. Lee, Macromol. Res., 2016, 24, 197–204 CrossRef CAS.
- H. Kim and C. Leal, ACS Nano, 2015, 9, 10214–10226 CrossRef CAS PubMed.
- G. Zhen, T. M. Hinton, B. W. Muir, S. Shi, M. Tizard, K. M. McLean, P. G. Hartley and P. Gunatillake, Mol. Pharm., 2012, 9, 2450–2457 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |