A highly effective Ni-modified MnOx catalyst for total oxidation of propane: the promotional role of nickel oxide
Yujie Xie,
Yun Guo,
Yanglong Guo,
Li Wang,
Wangcheng Zhan,
Yunsong Wang,
Xueqing Gong and
Guanzhong Lu*
Key Laboratory for Advanced Materials, Research Institute of Industrial Catalysis, East China University of Science and Technology, Shanghai 200237, P. R. China. E-mail: gzhlu@ecust.edu.cn; Fax: +86-21-64252923
First published on 12th May 2016
Abstract
Nickel promoted manganese oxide (MnNiOx) was prepared by the co-precipitation method and used as a catalyst for propane deep oxidation. The results show that the doping of Ni can effectively improve the catalytic performance of MnOx for propane total oxidation, and when Ni/Mn is 0.2, the MnNi0.2Ox catalyst exhibits the highest catalytic activity, for instance, the reaction temperature of 90% propane conversion (T90) was only ∼240 °C, and it demonstrates good thermal stability in the operation at temperatures alternating between 220 °C and 350 °C. It was found that an Mn–Ni–O solid solution can be formed by adding a moderate Ni amount into MnOx, resulting in changes in catalytically active sites by the synergetic interaction of Mn–Ni, such as a higher surface concentration of Mn4+ and oxygen vacancies, higher oxygen mobility and better reducibility. And the outstanding catalytic property of MnNi0.2Ox can be also related to its high surface area. Furthermore, the in situ DRIFT technique was used to investigate the reaction process of propane oxidation over the MnOx and MnNi0.2Ox catalysts, and the results suggest that the reaction mechanism is hardly changed after Ni doping in MnOx.
1. Introduction
The release of volatile organic compounds (VOCs) to the atmosphere has resulted in serious environmental problems in recent years, and environmental legislation has imposed increasingly stringent standards for VOC emissions. The prevalent VOCs, linear short chain alkanes, one of the largest fractions of hydrocarbons (HCs) made from automobile exhausts, are some of the most difficult to eliminate, due to their use as transportation fuels as well as being essential feed stocks for chemical production.1 Air pollution caused by these automotive exhausts is expected to worsen as the demand for privately owned vehicles increases.2 Although physical adsorption is an appropriate and convenient way to remove them to some extent, in this case the pollution can easily be transferred onto a solid which must then be disposed of as chemical waste.3 To remove VOCs, catalytic oxidation represents a promising and low-cost technology as it provides the potential to eliminate pollutants totally to carbon dioxide and water.Liquefied Petroleum Gas (LPG, composed of primarily propane and butane), as an alternative cleaner burning fuel, is gaining ground for application in fuel-powered vehicles because of its attractive features, such as clean combustion, high energy-density, simple storage and being ready for use in transportation.4 However, tailpipe emissions from LPG fueled vehicles and other combustors such as LPG engines and burners still contain high amounts of hazardous HCs. Up to 80% of HC emissions are produced in the first 60 to 90 s following a cold-start, because of the catalytic converter’s inability to oxidize HCs at low temperatures of 200–300 °C.5 As a result, it is quite essential to develop efficient catalysts that can completely remove propane emitted from LPG combustors, which is more difficult to eliminate than butane at low concentrations.6
Generally speaking, 75% of the catalysts used for VOC destruction are noble metal catalysts. Palladium and platinum based catalysts are strongly active even at relatively low temperature and have been studied thoroughly.7 For propane total oxidation, supported palladium and platinum catalysts are found to possess high activity and thermal stability.8–12 Nevertheless, they have some disadvantages like high sintering rates, volatility, poisoning in the presence of water or sulfur compounds and high price.13 Hence many efforts have been directed towards transition metal oxides as effective and economical catalysts for the catalytic combustion of alkanes in recent years. These oxide materials are less costly, more resistant to poisoning and, in certain cases, they exhibit catalytic activity comparable to that of noble metal catalysts. Much attention has been paid to perovskites, spinels, hydrotalcites and some single-oxide catalysts.14–18
Manganese oxides are reported to be one of the most efficient and environmentally friendly oxidation catalysts for catalytic combustion.19 Different polymorphs of MnO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and Mn3O4
20 and Mn3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 were reported to be active and stable catalysts for the oxidation of some organic compounds. Compared with single component oxide catalysts, MnOx mixed oxides modified with other metal oxides exhibit higher catalytic activity for some combustion reactions, which might be attributed to the capability of manganese to form oxides with different oxidation states and its high oxygen storage capacity.22 It was reported that the single oxides of Ni, Co, Fe and Mn have high activity for the decomposition of ozone, and mixed 3d-transition metal oxides were proved to be more active than the single ones.23 Hopcalite (CuMnOx), one of the most widely used and long-standing mixed oxide catalysts discovered around 90 years ago,24 has been mainly employed for low temperature CO oxidation22 and propane oxidation.25 Solsona et al. observed that a co-precipitated CuMnOx catalyst was more active than supported palladium catalysts, and its activity was further improved by the incorporation of gold nanoparticles into the CuMnOx structure.26 The preparation method can seriously affect the surface phase composition and physico-chemical properties of a catalyst, which may finally influence the catalytic activity. As a conventional preparation method of catalysts and inorganic materials, co-precipitation has been widely applied in the preparation of composite oxides, because of its simple synthesis process, short preparation period, low cost and good repeatability. The experimental variables can be easily controlled to fully optimize the preparation conditions, and the obtained materials are usually in a uniform chemical composition. Venkataswamy et al.27 used the co-precipitation method to prepare a nanostructured CeMn mixed oxide catalyst for CO oxidation, and found that the presence of moderate Mn in CeO2 led to the formation of solid solutions and the strong synergetic interaction of Mn–Ce, which is in favor of the improvement of reducibility and activation of surface oxygen, and the acceleration of reactant gas adsorption.28
21 were reported to be active and stable catalysts for the oxidation of some organic compounds. Compared with single component oxide catalysts, MnOx mixed oxides modified with other metal oxides exhibit higher catalytic activity for some combustion reactions, which might be attributed to the capability of manganese to form oxides with different oxidation states and its high oxygen storage capacity.22 It was reported that the single oxides of Ni, Co, Fe and Mn have high activity for the decomposition of ozone, and mixed 3d-transition metal oxides were proved to be more active than the single ones.23 Hopcalite (CuMnOx), one of the most widely used and long-standing mixed oxide catalysts discovered around 90 years ago,24 has been mainly employed for low temperature CO oxidation22 and propane oxidation.25 Solsona et al. observed that a co-precipitated CuMnOx catalyst was more active than supported palladium catalysts, and its activity was further improved by the incorporation of gold nanoparticles into the CuMnOx structure.26 The preparation method can seriously affect the surface phase composition and physico-chemical properties of a catalyst, which may finally influence the catalytic activity. As a conventional preparation method of catalysts and inorganic materials, co-precipitation has been widely applied in the preparation of composite oxides, because of its simple synthesis process, short preparation period, low cost and good repeatability. The experimental variables can be easily controlled to fully optimize the preparation conditions, and the obtained materials are usually in a uniform chemical composition. Venkataswamy et al.27 used the co-precipitation method to prepare a nanostructured CeMn mixed oxide catalyst for CO oxidation, and found that the presence of moderate Mn in CeO2 led to the formation of solid solutions and the strong synergetic interaction of Mn–Ce, which is in favor of the improvement of reducibility and activation of surface oxygen, and the acceleration of reactant gas adsorption.28
Herein, we want to develop low light-off oxidation catalysts for eliminating HC emissions, especially the emission of propane which is the main component in LPG. The conventional co-precipitation method was used to prepare a highly effective Ni-modified MnOx catalyst for propane total oxidation, in order to realize the industrial application of this catalyst. The effect of Ni on the catalytic properties of MnOx was investigated in detail. The results show that the MnNi0.2Ox catalyst showed very high activity for propane deep oxidation compared with the single MnOx. The reasons for MnNi0.2Ox having the highest catalytic activity for propane combustion were illuminated and discussed.
2. Experimental section
2.1. Catalyst preparation
MnNiOx catalysts were synthesized by the co-precipitation method in aqueous solutions, in which manganese nitrate (Mn(NO3)2, AR grade) and nickel nitrate (Ni(NO3)2·6H2O, AR grade) were used as the precursors. The desired amounts of the precursors were solved and mixed in deionized water under stirring. An aqueous solution of Na2CO3 was added dropwise into the synthesis solution above until pH = 8.5. After it was aged for 14 h at 80 °C under stirring, the obtained precipitate was filtered and washed with deionized water to remove Na+ ions possibly left in the precipitate. Then the obtained solid was dried at 120 °C in air overnight, and calcined at 300–500 °C for 4 h in a muffle furnace. Pure MnOx and NiO were prepared with the same procedures as the MnNiOx mixed oxide catalysts.2.2. Characterization techniques
The surface areas of the catalysts were measured by N2 adsorption at −196 °C on a Micromeritics ASAP 2020 apparatus and calculated by the Brunauer–Emmett–Teller (BET) method. Powder X-ray diffraction (XRD) patterns were recorded on a Brook D8 Focus XRD diffraction spectrometer with Cu Kα radiation (λ = 0.154 nm, operated at 40 kV and 40 mA). Laser Raman spectra were measured on a Renishaw inVia-Reflex Micro-Raman spectroscopy system at ambient conditions and the 514 nm line of a Spectra Physics Ar+ laser was used for excitation. Scanning electron microscopy (SEM) images were taken on a Hitachi S-3400N scanning electron microscope operated at 15 kV, and the sample was covered with a thin layer of gold before testing. Transmission electron microscopy (TEM) images were obtained on a JEOL JEM-2100 microscope operated at 200 kV. The sample to be measured was dispersed in ethanol and then collected onto copper grids covered with carbon films. The grid was loaded into the microscope after evaporation of the liquid phase. X-ray photoelectron spectroscopy (XPS) spectra of samples were obtained on a Kratos Axis Ultra-DLD photoelectron spectrometer equipped with Al Kα (1486.6 eV) radiation as the excitation source. The sample to be tested was pre-treated in N2 at 500 °C for 30 min. All the binding energies (BE) were determined with respect to the C 1s line (284.8 eV) originating from adventitious carbon.Hydrogen temperature programmed reduction (H2-TPR) was performed in a quartz U-tube with 100 mg of catalyst in a conventional flow system equipped with a thermal conductivity detector (TCD). The reducing gas consisted of 5% H2/Ar (45 ml min−1) and the heating rate was 10 °C min−1.
Temperature programmed desorption of oxygen (O2-TPD) was performed in a quartz U-tube reactor system equipped with an online Hiden Analytical HAL301 mass spectrometer. Prior to each test, 100 mg of the sample was pre-treated in vol 3% O2/He mixture gas at 400 °C for 1 h. After cooling down to room temperature, pure He with a flow rate of 30 ml min−1 instead of vol 3% O2/He was purged into the reactor until stabilization of the MS baseline. The reactor was heated at 10 °C min−1 from room temperature to 850 °C. The signal of desorbed oxygen (m/z = 32) was collected using an MS detector.
The in situ DRIFT spectra of propane were recorded on a Nicolet 6700 FT-IR spectrometer equipped with an MCT detector. In the DRIFT cell with ZnSe windows connected with a gas flow system, the catalyst was pretreated in Ar at 400 °C for 1 h, and then the catalyst was cooled down to 50 °C for propane adsorption. The background spectra were recorded at certain temperatures during the cooling. Then the Ar gas was replaced by the reaction mixture gas of 2000 ppm C3H8 and 5% O2 balanced by Ar with a total flow rate of 50 ml min−1. After the reaction in the cell was stabilized for 1 h at each temperature, the DRIFT spectra were obtained and background spectrum subtracted.
2.3. Catalytic performance testing
The catalytic activity of the sample for the total oxidation of propane was examined in a fixed bed reactor. 200 mg of catalyst was placed in a quartz tube reactor and the feed gas consisted of 0.2% C3H8 and 5% O2 in Ar balance gas. The total flow rate was controlled to 100 ml min−1 and the gas hourly space velocity (GHSV) was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The reactants and products were analyzed online using a gas chromatograph (GC 2060 system) equipped with an FID. The catalytic activities were described as propane conversion (%), and T10, T50 and T90, which are the reaction temperatures for the propane conversions of 10, 50, and 90%, respectively.
000 h−1. The reactants and products were analyzed online using a gas chromatograph (GC 2060 system) equipped with an FID. The catalytic activities were described as propane conversion (%), and T10, T50 and T90, which are the reaction temperatures for the propane conversions of 10, 50, and 90%, respectively.
2.4. Reaction kinetics testing
The kinetics parameters for propane oxidation were measured in the fixed-bed reactor as mentioned above, and the catalytic reaction data were obtained after the reaction was stable for 1 h, and the propane conversion was controlled to <15%. The reaction rate (r, mol (gcat s)−1) of propane conversion was calculated according to the following eqn (1),
 | (1) |
When the conversion of propane is lower than 15%, the dependence of the reaction rate (r) on the products of CO2 and H2O can be ignored. Therefore, the empirical kinetic expression of the reaction rate equation can then be described by eqn (2),
 | (2) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r = ln r = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A + αln A + αln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC3H8 + βln PC3H8 + βln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO2 − Ea/RT PO2 − Ea/RT
| (3) |
As the conversion of propane is lower than 15% during the kinetics data testing, the change of feed gas may be approximately ignored. Hence, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A, αln
A, αln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC3H8 and βln
PC3H8 and βln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO2 are supposed to be approximately constant, and eqn (3) can be simplified to eqn (4).
PO2 are supposed to be approximately constant, and eqn (3) can be simplified to eqn (4).
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r = −Ea/RT + C r = −Ea/RT + C
| (4) |
Thus the activation energy (Ea) of this catalytic reaction can be obtained from the slope of the resulting linear plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r versus 1/T.
r versus 1/T.
3. Results and discussion
3.1. Catalytic performance and kinetic parameters for propane oxidation
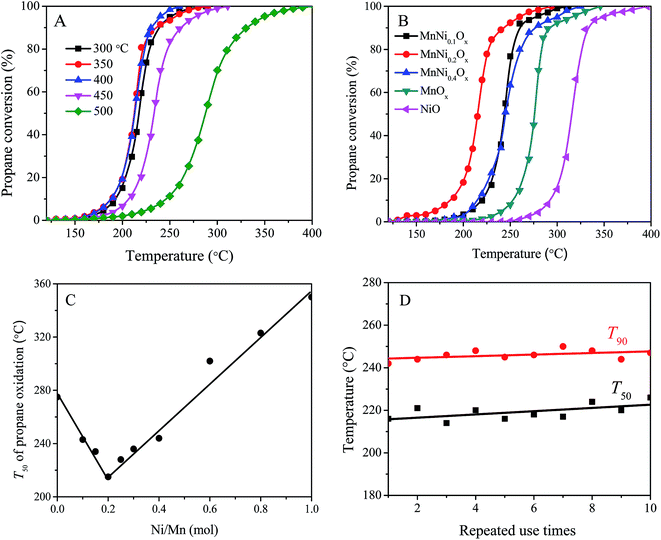
Since only CO2 was detected in all the reaction processes, the MnNiOx catalysts possessed excellent selectivity for propane deep oxidation. Firstly, the effect of the calcination temperature (300–500 °C) on the catalytic activity of the MnNi0.2Ox sample was investigated, and the catalytic activities for propane combustion are shown in Fig. 1A. The results show that the calcination temperature obviously affected the catalytic activity of MnNi0.2Ox and the samples calcined at lower temperature (300–400 °C) exhibited higher catalytic activity for propane total oxidation. Unless otherwise indicated, the calcination temperature of the catalyst was 400 °C hereinafter. For the MnNiOx catalysts calcined at 400 °C, the effect of the Ni amount on the catalytic activities of the MnNiOx catalysts for propane total oxidation are shown in Fig. 1B and their T10 (the reaction temperature of 10% propane conversion), T50, and T90 are listed in Table 1. The results show that the catalytic activities of the MnNiOx catalysts are affected obviously by the Ni/Mn molar ratio, and the activities of the MnNiOx catalysts are ranked as follows: MnNi0.2Ox > MnNi0.1Ox ≥ MnNi0.4Ox > MnOx > NiO. Apparently, the presence of Ni can improve the catalytic activity of an MnOx sample, compared with the single oxide of MnOx and NiO. As shown in Fig. 1C, when the molar ratio of Ni/Mn is 0.2 the MnNi0.2Ox sample possesses the best catalytic performance, and upon further increasing the Ni amount in the MnNiOx catalyst, its catalytic activity would contrarily be reduced. The T10, T50 and T90 values over the MnNi0.2Ox catalyst are 186, 215 and 242 °C for propane deep oxidation, respectively, which are 67, 61 and 52 °C lower than those over pure MnOx. The re-usability of the MnNi0.2Ox was tested and the results are shown in Fig. 1D. The results show that after being repeatedly used ten times for propane oxidation, T50 and T90 on the catalyst still kept around 220 °C and 240 °C. No obvious deactivation was observed, which shows that the MnNi0.2Ox catalyst exhibits favorable re-usability.| Sample | T10 (°C) | T50 (°C) | T90 (°C) | SBET (m2 g−1) | r × 105 (mol (gcat s)−1) | Ea (kJ mol−1) |
|---|---|---|---|---|---|---|
| MnNi0.1Ox | 221 | 242 | 258 | 82 | 20.7 | 106 |
| MnNi0.2Ox | 186 | 215 | 242 | 111 | 21.5 | 67 |
| MnNi0.4Ox | 214 | 243 | 277 | 97 | 19.5 | 114 |
| MnOx | 253 | 276 | 294 | 88 | 7.0 | 128 |
| NiO | 294 | 315 | 333 | 136 | 0.6 | 166 |
It was reported that using a traditional CuMn catalyst propane could be totally oxidized at >300 °C, and the T90 of propane oxidation over supported Co3O4 also reached as high as 260 °C.24,26,29 Comparing with the typical noble metal catalysts (such as supported Pt and Pd catalysts), the activity of the MnNi0.2Ox catalyst is quite close or even much better.30–33 And the MnNi0.2Ox catalyst exhibited much better operational stability than some of the catalysts reported. Therefore, this MnNi0.2Ox catalyst is a potential catalyst for VOC oxidation.
The reaction rates of propane oxidation over the MnNiOx catalysts at 270 °C were measured, and the results are shown in Table 1. The results show that the MnNi0.2Ox catalyst has the highest rate of 20.7 × 10−5 mol (gcat s)−1, almost three times higher than that of the MnOx (7.0 × 10−5 mol (gcat s)−1). Fig. 2 displays Arrhenius plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r versus 1/T for all the samples, and the activation energy (Ea) was obtained from the slope of the linear plot and is listed in Table 1. Obviously, the Ea values of MnNiOx samples are lower than that of MnOx, in which the MnNi0.2Ox sample exhibits the lowest Ea (67 kJ mol−1).
r versus 1/T for all the samples, and the activation energy (Ea) was obtained from the slope of the linear plot and is listed in Table 1. Obviously, the Ea values of MnNiOx samples are lower than that of MnOx, in which the MnNi0.2Ox sample exhibits the lowest Ea (67 kJ mol−1).
3.2. Thermal stability of MnNi0.2Ox and MnOx
Fig. 3A shows propane conversion as a function of the reaction time at different temperatures over the MnNi0.2Ox and MnOx catalysts. It can be found that the catalytic activities of the two samples are hardly changed after 50 h of the reaction, in which the reaction temperature for MnNi0.2Ox was 240 °C and that for MnOx was 290 °C, and the propane conversions can be kept at ∼90%. To further examine the thermal stability of the catalyst, an accelerated aging process by cycling the reaction temperature was carried out for each catalyst. As shown in Fig. 3B, the catalysts were tested under alternating temperature between 220 °C (270 °C) for 10 h and 350 °C for 10 h over MnNi0.2Ox (or MnOx), and the catalytic activities of both catalysts hardly varied for propane oxidation. These results suggest that MnOx and MnNi0.2Ox demonstrate favorable thermal stability, and the propane conversion over MnNi0.2Ox is higher than that over MnOx. | ||
| Fig. 3 Propane conversion as a function of the reaction time at different temperatures over the MnNi0.2Ox and MnOx catalysts. | ||
3.3. BET surface area and XRD analyses
Table 1 lists the BET surface areas (SBET) of MnNiOx samples with different molar ratios of Ni/Mn obtained by low temperature N2 adsorption. Among all samples NiO has the largest surface area (∼136 m2 g−1), and among the MnNi mixed oxides, MnNi0.2Ox shows the largest specific surface area of ∼111 m2 g−1. In general, for a solid catalyst a larger specific surface area can support more active sites, thus the highest catalytic activity of MnNi0.2Ox among the MnNiOx samples is partially attributed to its largest surface area. However, the surface area of MnNi0.1Ox is hardly changed compared with MnOx, but the activity of MnNi0.1Ox is obviously higher than that of MnOx, which shows that the surface area is only one of the main factors affecting the catalytic activity rather than the sole factor. The presence of Ni in MnNiOx not only affects the surface area of MnOx, but also varies the nature of the active sites, the properties of surface oxygen species (including oxygen vacancies and oxygen mobility), reducibility of the catalyst, and surface concentration of Mn4+, etc. The combination of these factors leads to the improvement of the catalytic performance of the MnNi0.2Ox catalyst.The powder XRD patterns of the prepared catalysts are shown in Fig. 4. The nickel oxide sample showed primarily the diffraction peaks of the bunsenite phase of NiO (JCPDS 47-1049), and manganese oxide hardly exhibited the diffraction peaks, that is to say, the MnOx sample is amorphous. After adding Ni into MnOx, part of the Mn species still existed as amorphous MnOx, and the diffraction peaks of NiO could be hardly observed. When doping a small amount of Ni, there are diffraction peaks of MnCO3 in the XRD spectrum of the MnNi0.1Ox catalyst, possibly owing to incomplete decomposition. With an increase in the Ni content, the diffraction peaks of new phases attributed to Ni6MnO8 (JCPDS 83-1186) and NiMnO3 (JCPDS 75-2089) mixed oxides can be observed. Ni6MnO8 contains Mn4+ species which can supply oxygen vacancies.34 Thus, MnNiOx catalysts with moderate Ni amounts are expected to contain more oxygen vacancies, due to the formation of an Mn–Ni–O (as Ni6MnO8) solid solution.
3.4. Raman spectroscopy
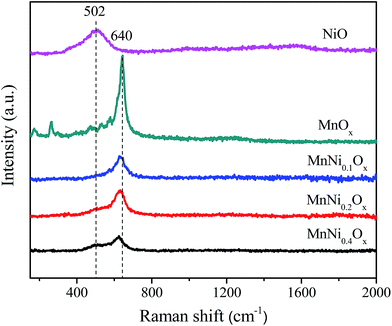
Raman spectra of MnNiOx catalysts are shown in Fig. 5. For the MnOx sample, the strong peak at 640 cm−1 is assigned to the stretching vibration of the metal–oxygen chain of Mn–O–Mn,35 and NiO shows a broad peak at ∼502 cm−1 which is attributed to the Ni–O stretching mode.36 In the Raman spectra of MnNiOx, only a vibration peak of MnOx can be observed when the Ni amount is less. With the increase in the Ni amount, the Raman peak of NiO became gradually visible and the peak of MnOx slightly shifted to lower wave numbers, which means a sensitive indication of defective structures. Generally speaking, Raman frequency shift is dependent mainly on oxygen vacancies and lattice reconfiguration. Therefore, the addition of Ni can cause the formation of oxygen vacancies and deformation of the MnOx structure through the synergistic effect of Mn and Ni species, which finally affects the nature of catalytically active sites.3.5. SEM and TEM images
Fig. 6 exhibits the SEM images of the MnNiOx catalysts. For single oxides, MnOx shows an agglomerated bulk of globular particles with blurred inter-particle boundaries37 and NiO is composed of irregular polyhedra. After adding a small amount of Ni to MnOx, the MnNi0.1Ox sample consists of a few smaller globular particles besides some larger particles with a clear boundary like the MnOx sample. Interestingly, when the Ni/Mn molar ratio is increased, in the particle surface of MnNi0.2Ox there are many fiber-like nanowires or nano-leaves, and its particle size is 3–4 times larger than the size of the MnNi0.1Ox sample. And these attached fibers on the MnNi0.2Ox catalyst can be considered to be one of the reasons for its higher surface area. However, these fibers disappeared after adding a further Ni amount. And MnNi0.4Ox has similar particles to MnOx but these particles can be clearly separated. The results above suggest that doping Ni into MnOx with an appropriate amount can bring visible morphological changes.TEM images of MnOx and MnNi0.2Ox catalysts are shown in Fig. 7. In the TEM image of MnOx, many crystallites with irregular diameters formed the assembling particles. Two different features can be detected for the MnNi0.2Ox sample. As shown in MnNi0.2Ox (A), crystallites with denser and smaller spherical particles than MnOx were observed; when changing the scanning area, the image labeled as MnNi0.2Ox (B) was obtained, in which ribbon-like nanowires with lengths of 20–140 nm were clearly observed, in accord with its surface fibers shown in the SEM image.
3.6. H2-TPR
The reduction behavior of MnNiOx catalysts was investigated and the results are shown in Fig. 8. Pure NiO has only one wide peak at ∼400 °C, assigned to the reduction of NiO to Ni.38 The MnOx sample exhibited two well-defined reduction peaks at ∼285 °C (peak α) and ∼415 °C (peak β), which can be attributed to the reduction of MnOx to Mn3O4 and the reduction of Mn3O4 to MnO, respectively.39,40 When doping a lesser amount of Ni in MnOx, the α and β peaks of the MnNi0.1Ox catalyst noticeably shifted to lower temperatures accompanying the decrease in their areas, and the reduction peak of NiO was hardly observed. With the increase in the Ni content, the top temperatures of peak α and β shifted slightly to lower temperatures and their areas further decreased, and the reduction peak of NiO gradually appeared. The results above show that the reduction peaks of both Mn and Ni species moved to lower temperatures for the MnNi mixed oxides with higher Ni content (as MnNi0.2Ox and MnNi0.4Ox). Generally, the redox cycles of Mn and Ni oxides are connected with gaseous oxygen activation. Since the sizes of metal ions (such as Mn4+, Mn3+, Mn2+, and Ni2+) are quite different, a strong interaction between them can be created to realize co-existence of these ions.41 After a suitable distribution of metal components was formed by a solid reaction, the Mn–Ni–O solid solutions would be formed, which would affect the activity of related bonded oxygen. The dropping of the reduction temperature should be ascribed to the synergistic effect between the Mn and Ni species, due to the formation of a solid solution, in which the mobility of oxygen species can be effectively promoted, resulting in the production of more active oxygen species for the reaction.42 Therefore, doping Ni into MnOx can produce more active oxygen species in the MnNiOx composite oxide through the strong synergistic effect between the Mn and Ni species, and improve the reducibility of this catalyst at low temperature.3.7. O2-TPD
Fig. 9 shows the O2-TPD curves of the MnNiOx samples. The desorption peak was hardly observed in the O2-TPD curve of pure NiO, suggesting little oxygen release. For pure MnOx, the middle-temperature (MT) desorption peaks located at 350–650 °C can be attributed to the release of lattice oxygen, and the high-temperature (HT) peaks at >650 °C are related to the decomposition of bulk MnOx.43 But the desorption peaks of surface adsorbed oxygen (O2, O2− and O−), which tend to be released easily, were hardly found at <350 °C on the MnOx sample.44,45 After adding Ni, the MT peaks of the MnNi0.1Ox catalyst partially merged (three peaks merged to two peaks), indicating mobility of lattice oxygen by the aid of an interaction between Mn and Ni species. With an increase in the Ni content, the HT desorption peaks rapidly became invisible, from which we can infer that less bulk MnOx existed in MnNi mixed oxides with higher Ni content and the lattice oxygen was mainly generated from Mn–Ni–O solid solutions. Compared with MnNi0.4Ox, in addition, the area and intensity of the MT desorption peak of MnNi0.2Ox are a bit higher, suggesting better desorption ability and mobility of lattice oxygen over the MnNi0.2Ox catalyst.46 Since the deep oxidation of propane over the MnOx catalyst follows the Mars-van Krevelen mechanism,47 the increase in the mobility and activity of lattice oxygen species of the MnNiOx catalyst is beneficial to improve its catalytic activity.3.8. XPS analysis
Fig. 10 shows the XPS Mn 2p and O 1s spectra of MnOx and MnNi0.2Ox catalysts, and surface atom ratios of Mn3+/Mn2+, Mn4+/Mn3+ and Oads/Olatt calculated from the XPS spectra using XPSPEAK 4.1 are listed in Table 2. The asymmetrical Mn 2p3/2 XPS signal at binding energy (BE) of ∼641 eV can be decomposed to three Gaussian–Lorentzian peaks at ∼640.6, 641.8 and 643 eV, which are ascribable to the surface species of Mn2+, Mn3+ and Mn4+, respectively.48,49 After adding Ni0.2 into MnOx, the atomic ratio of Mn3+/Mn2+ decreased from 1.85 (MnOx) to 1.35 (MnNi0.2Ox), and the atomic ratio of Mn4+/Mn3+ increased from 0.48 to 0.92. This is because the presence of Ni in the MnNi0.2Ox solid solution can effectively restrain the diffusion of ions (such as Mnn+ and O2+) and inhibit the transformation of Mn4+ to Mn3+, resulting in an increase in the Mn4+ concentration.50| Sample | BE of Mn 2p (eV) | BE of O 1s (eV) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mn2+ | Mn3+ | Mn4+ | Mn3+/Mn2+ | Mn4+/Mn3+ | Olatt | Oad | Oad/Olatt | |
| MnOx | 640.6 | 641.8 | 643.2 | 1.85 | 0.48 | 529.7 | 531.2 | 0.72 |
| MnNi0.2Ox | 640.7 | 641.9 | 643.2 | 1.35 | 0.92 | 529.8 | 531.2 | 1.39 |
The O 1s peaks can also be deconvoluted to three peaks: the lowest BE peak at ∼529.7 eV is assigned to the lattice oxygen (Olatt), the peak at BE of ∼531.2 eV is attributed to the surface adsorbed oxygen (Oads), and the high BE peak at ∼532.5 eV is associated with adsorbed OH groups or adsorbed molecular water.50–52 The results show that the Oads/Olatt ratio of MnNi0.2Ox is 1.39, and much higher than that of MnOx (0.72). It was reported that when the Mn species existed as Mn4+–Oads Lewis acid–base pairs, Mn4+ can be better dispersed on the surface, thus the catalyst with a higher Mn4+/Mn3+ ratio can contain more adsorbed oxygen species.53,54 Therefore, compared with pure MnOx, the MnNi0.2Ox catalyst possesses a higher Mn4+/Mn3+ atomic ratio and contains more adsorbed oxygen species, which is beneficial to the improvement of its catalytic oxidation performance.
3.9. In situ DRIFT spectroscopy
Fig. 11 shows the in situ DRIFT spectra of MnOx and MnNi0.2Ox catalysts at 1000–4000 cm−1 and 50–300 °C under an atmosphere of 2000 ppm C3H8 + 5 vol% O2/Ar (50 ml min−1). The IR spectra were recorded after 1 h of the reaction at each temperature. In these IR spectra, the band at 2969 cm−1 and shoulder at 2900 cm−1 correspond to C–H vibrations of gaseous propane, and the bands located at 1300–1700 cm−1 were ascribed to various carboxylate species such as formate (1590 cm−1), acetate (1575, 1430 and 1433 cm−1) and mono- or poly-dentate carbonate (1350 cm−1).55,56 Besides, the band at ∼3500 cm−1 on MnNi0.2Ox was owing to combined OH groups on the surface.57 When the reaction temperature was increased from 50 to 200 °C, the carboxylate peaks gradually became weaker and finally disappeared, implying the partial oxidation of propane over the MnOx and MnNi0.2Ox catalysts, in which labile carboxylates should be the oxygenated intermediates. At 300 °C, the IR spectra of the surfaces of both catalysts became very weak, reflecting the deep oxidation of propane. It is interesting to find that the Ni-modified sample possessed obvious carboxylate peaks at a lower wavenumber (1575 cm−1) compared with the IR spectra of MnOx (1590 cm−1), possibly resulting from the enhanced adsorption of reactants.58 Therefore, it can be speculated that Ni modification hardly changes the reaction mechanism of propane deep oxidation, and only the nature of surface carbonate species is varied by the presence of Ni, which improved the property of the active sites for propane adsorption during the preliminary oxidation process, accelerating the activation of propane gas. | ||
| Fig. 11 In situ DRIFT spectra of MnOx and MnNi0.2Ox catalysts under an atmosphere of 2000 ppm C3H8 + 5 vol% O2/Ar (50 ml min−1) at 50–300 °C. | ||
4. Conclusions
In summary, the MnNiOx catalysts synthesized by the co-precipitation method exhibited excellent catalytic performance for the combustion of propane. The doping of Ni into MnOx apparently enhanced the catalytic activity, and the best molar ratio of Ni/Mn was 0.2. Using the MnNi0.2Ox catalyst, T90 (the reaction temperature of 90% propane conversion) and T50 were ∼240 °C and 215 °C respectively. At 270 °C, the reaction rate on the MnNi0.2Ox catalyst reached 20.7 × 10−5 mol (gcat s)−1, which was three times higher than that on MnOx. The MnNi0.2Ox also demonstrated good thermal stability for propane total oxidation, and no deactivation was observed after online testing as long as 50 h, at temperatures alternating between 220 °C for 10 h and 350 °C for 10 h.The addition of Ni can promote the formation of oxygen vacancies and deformation of the MnOx structure through the synergistic effect of Mn and Ni species, which affects the nature of catalytically active sites, but a lesser Ni doping amount was not able to change the nature of the active sites. When the introduction of Ni into MnOx reaches Ni/Mn > 0.2, an Mn–Ni–O (as Ni6MnO8) solid solution can form in the MnNiOx sample, resulting in an increase in the Mn4+ content, amount of oxygen vacancies, oxygen mobility and reduction property, which can effectively improve its catalytic performance for propane total oxidation. Contrarily, the presence of excess Ni content would weaken its promotional role for the MnOx catalyst. The presence of Ni in the MnNiOx sample hardly changes the reaction mechanism of propane deep oxidation, and only the nature of surface carbonate species is varied on the surface, which improves the property of the active sites for propane adsorption during the preliminary oxidation process, accelerating the activation of propane gas.
Acknowledgements
This work was supported financially by the National High Technology Research and Development Program of China (2012AA111717), the Commission of Science and Technology of Shanghai Municipality (15DZ1205305) and Fundamental Research Funds for the Central Universities (WJ1514020).References
- T. V. Choudhary, S. Banerjee and V. R. Choudhary, Appl. Catal., A, 2002, 234, 1–23 CrossRef CAS.
- J. A. Enterkin, W. Setthapun, J. W. Elam, S. T. Christensen, F. A. Rabuffetti, L. D. Marks, P. C. Stair, K. R. Poeppelmeier and C. L. Marshall, ACS Catal., 2011, 1, 629–635 CrossRef CAS.
- D. P. Debecker, B. Farin, E. M. Gaigneaux, C. Sanchez and C. Sassoye, Appl. Catal., A, 2014, 481, 11–18 CrossRef CAS.
- Z. Jiang, T. Xiao, V. L. Kuznetsov and P. P. Edwards, Philos. Trans. R. Soc., A, 2010, 368, 3343–3364 CrossRef CAS PubMed.
- R. M. Heck and R. J. Farrauto, Appl. Catal., A, 2001, 221, 443–457 CrossRef CAS.
- V. R. Choudhary, S. Banerjee and S. G. Pataskar, Appl. Catal., A, 2003, 253, 65–74 CrossRef CAS.
- L. F. Liotta, Appl. Catal., B, 2010, 100, 403–412 CrossRef CAS.
- L. Zhang, D. Weng, B. Wang and X. D. Wu, Catal. Commun., 2010, 11, 1229–1232 CrossRef CAS.
- M. Y. Kim, S. M. Park, G. Seo and K. S. Song, Catal. Lett., 2010, 138, 205–214 CrossRef CAS.
- J. E. Park, K. B. Kim, K. W. Seo, K. S. Song and E. D. Park, Res. Chem. Intermed., 2011, 37, 1135–1143 CrossRef CAS.
- M. N. Taylor, W. Zhou, T. Garcia, B. Solsona, A. F. Carley, C. J. Kiely and S. H. Taylor, J. Catal., 2012, 285, 103–114 CrossRef CAS.
- M. Taylor, E. N. Ndifor, T. Garcia, B. Solsona, A. F. Caley and S. H. Taylor, Appl. Catal., A, 2008, 350, 63–70 CrossRef CAS.
- G. Busca, M. Daturi, E. Finocchio, V. Lorenzelli, G. Ramis and R. J. Willey, Catal. Today, 1997, 33, 239–249 CrossRef CAS.
- R. Ran, D. Weng, X. D. Wu, J. Fan and L. Qing, Catal. Today, 2007, 126, 394–399 CrossRef CAS.
- K. Rida, A. Benabbas, F. Bouremmad, M. A. Peña, E. Sastre and A. Martínez-Arias, Appl. Catal., B, 2008, 84, 457–467 CrossRef CAS.
- U. Zavyalova, B. Nigrovski, K. Pollok, F. Langenhorst, B. Müller, P. Scholz and B. Ondruschka, Appl. Catal., B, 2008, 83, 221–228 CrossRef CAS.
- J. Cheng, J. J. Yu, X. P. Wang, L. D. Li, J. J. Li and Z. P. Hao, Energy Fuels, 2008, 22, 2131–2137 CrossRef CAS.
- V. G. Milt, M. A. Ulla and E. A. Lombardo, Catal. Lett., 2000, 65, 67–73 CrossRef CAS.
- L. F. Liotta, G. Di Carlo, G. Pantaleo, A. M. Venezia and D. G. eganello, Appl. Catal., B, 2006, 66, 217–227 CrossRef CAS.
- T. García, B. Solsona and S. H. Taylor, Appl. Catal., B, 2006, 66, 92–96 CrossRef.
- B. Solsona, I. Vázquez, T. Garcia, T. Davies and S. Taylor, Catal. Lett., 2007, 3–4, 116–121 CrossRef.
- M. R. Morales, B. P. Barbero and L. E. Cadús, Appl. Catal., B, 2007, 74, 1–10 CrossRef CAS.
- D. Mehandjiev, A. Naydenov and G. Ivanov, Appl. Catal., A, 2001, 206, 13–18 CrossRef CAS.
- M. R. Morales, B. P. Barbero and L. E. Cadús, Appl. Catal., B, 2006, 67, 229–236 CrossRef CAS.
- M. Baldi, V. S. Escribano, J. M. G. Amores, F. Milella and G. Busca, Appl. Catal., B, 1998, 17, 175–182 CrossRef.
- B. Solsona, T. Garcia, S. Agouram, G. J. Hutchings and S. H. Taylor, Appl. Catal., B, 2011, 101, 388–396 CrossRef CAS.
- P. Venkataswamy, K. N. Rao, D. Jampaiah and B. M. Reddy, Appl. Catal., B, 2015, 162, 122–132 CrossRef CAS.
- M. Machida, M. Uto, D. Kurogi and T. Kijima, Chem. Mater., 2000, 12, 3158–3164 CrossRef CAS.
- Z. Z. Zhu, G. Z. Lu, Z. Z. Zhang, Y. Guo, Y. L. Guo and Y. Q. Wang, ACS Catal., 2013, 3, 1154–1164 CrossRef CAS.
- Y. Zheng, Y. Zheng, Y. H. Xiao, G. H. Cai and K. M. Wei, Catal. Commun., 2013, 39, 1–4 CrossRef CAS.
- M. Li, D. Weng, X. D. Wu, J. Wan and B. Wang, Catal. Today, 2013, 201, 19–24 CrossRef CAS.
- G. Wang, M. Meng, Y. Q. Zha and T. Ding, Fuel, 2010, 89, 2244–2251 CrossRef CAS.
- M. Li, X. D. Wu, J. Wan, S. Liu, R. Ran and D. Weng, Catal. Today, 2015, 242, 322–328 CrossRef CAS.
- A. Machocki, T. Ioannides, B. Stasinska, W. Gac, G. Avgouropoulos, D. Delimaris, W. Grzegorczyk and S. Pasieczna, J. Catal., 2004, 227, 282–296 CrossRef CAS.
- G. Liu, R. L. Yue, Y. Jia, Y. Ni, J. Yang, H. D. Liu, Z. Wang, X. F. Wu and Y. F. Chen, Particuology, 2013, 11, 454–459 CrossRef CAS.
- W. Z. Wang, Y. K. Liu, C. K. Xu, C. L. Zheng and G. H. Wang, Chem. Phys. Lett., 2002, 362, 119–122 CrossRef CAS.
- M. V. Gallegos, L. R. Falco, M. A. Peluso, J. E. Sambeth and H. J. Thomas, Waste Manage., 2013, 33, 1483–1490 CrossRef CAS PubMed.
- L. Christel, A. Pierre and D. A. R. Abel, Thermochim. Acta, 1997, 306, 51–59 CrossRef CAS.
- R. Craciun, B. Nentwich, K. Hadjiivanou and H. Knözinger, Appl. Catal., A, 2003, 243, 67–79 CrossRef CAS.
- M. Ferradon, J. Carno, S. Jaras and E. Bjornbon, Appl. Catal., A, 1999, 180, 141–151 CrossRef.
- W. X. Tang, Y. Z. Deng, W. H. Li, J. Li, G. Liu, S. D. Li, X. F. Wu and Y. F. Chen, Catal. Sci. Technol., 2016, 6, 1710–1718 CAS.
- S. F. Tang, Y. G. Li, S. M. Huang, Y. D. Xu, H. Q. Zhu, J. G. Wang and W. J. Shen, Appl. Catal., B, 2006, 62, 265–273 CrossRef.
- G. S. Qi and W. Li, Catal. Today, 2015, 258, 205–213 CrossRef CAS.
- A. Bielanski and J. Haber, Catal. Rev.: Sci. Eng., 1979, 19, 1–41 CAS.
- E. C. Njagi, H. C. Genuino, C. K. King’ondu, C. K. S. Dharmarathna and S. L. Suib, Appl. Catal., A, 2012, 421, 154–160 CrossRef.
- G. L. Zhou, H. Lan, H. Wang, H. M. Xie, G. Z. Zhang and X. X. Zheng, J. Mol. Catal. A: Chem., 2014, 393, 279–288 CrossRef CAS.
- B. Solsona, T. E. Davies, T. Garcia, I. Vazquez, A. Dejoz and S. H. Taylor, Appl. Catal., B, 2008, 84, 176–184 CrossRef CAS.
- R. P. Gupta and S. K. Sen, Phys. Rev. B: Solid State, 1974, 10, 71–79 CrossRef CAS.
- B. Puértolas, A. Smith, I. Vázquez, A. Dejoz, A. Moragues, T. Garcia and B. Solsona, Chem. Eng. J., 2013, 229, 547–558 CrossRef.
- H. Shinjoh, J. Alloys Compd., 2006, 408–412, 1061–1064 CrossRef CAS.
- J. L. G. Fierro, Catal. Today, 1990, 8, 153–174 CrossRef CAS.
- L. González Tejuca, A. T. Bell, J. L. G. Fierro and M. A. Pena, Appl. Surf. Sci., 1988, 31, 301–316 CrossRef.
- Y. X. Liu, H. X. Dai, J. G. Deng, Y. C. Du, X. W. Li, Z. X. Zhao, Y. Wang, B. Z. Gao, H. G. Yang and G. S. Guo, Appl. Catal., B, 2013, 141, 493–505 CrossRef.
- W. X. Tang, X. F. Wu, S. D. Li, X. Shan, G. Liu and Y. F. Chen, Appl. Catal., B, 2015, 162, 110–121 CrossRef CAS.
- Y. B. He, H. B. Ji, J. H. Xu and L. F. Wang, J. Nat. Gas Chem., 2009, 18, 359–364 CrossRef CAS.
- X. D. Wu, Z. Li, D. Weng, S. Liu, Z. C. Si and J. Fan, J. Hazard. Mater., 2012, 225, 146–154 CrossRef PubMed.
- Z. Ren, Z. L. Wu, W. Q. Song, W. Xiao, Y. B. Guo, J. Ding, S. L. Suib and P. X. Gao, Appl. Catal., B, 2016, 180, 150–160 CrossRef CAS.
- J. Y. Luo, M. Meng, Y. Q. Zha and L. H. Guo, J. Phys. Chem. C, 2008, 112, 8694–8701 CAS.
| This journal is © The Royal Society of Chemistry 2016 |