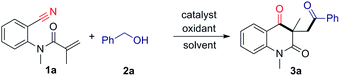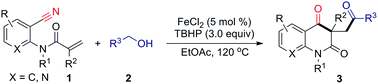Iron-catalyzed addition/cyclization cascade of activated alkenes with alcohols: access to carbonyl substituted quinoline-2,4-diones†
Shi-Sheng Wanga,
Hong Fua,
Guanlin Wanga,
Meng Sunb and
Ya-Min Li*a
aFaculty of Life Science and Technology, Kunming University of Science and Technology, Kunming 650500, P. R. China. E-mail: liym@kmust.edu.cn
bKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, Department of Chemistry & Materials Science, Northwest University, Xi'an 710127, P. R. China
First published on 24th May 2016
Abstract
A convenient and efficient iron-catalyzed radical addition/cyclization cascade of o-cyanoarylacrylamides with alcohols is presented for the synthesis of carbonyl substituted quinoline-2,4(1H,3H)-diones in moderate to excellent yields. This transformation exhibits a wide substrate scope and significant functional group tolerance.
Quinoline-2,4-dione scaffolds are available in a large number of natural products, pharmaceuticals and agrochemicals.1 For example, buchapine (I) shows significant anti-HIV activity in cell-based assays;1c compound II exhibits high binding affinity and selectivity for the 5-HT6 receptor;1d and quinoline dione III is found to be a highly potent and selective CB2R agonist1g (Fig. 1). Moreover, they often serve as valuable synthetic intermediates.2 Despite many strategies being developed to prepare quinoline-2,4-diones,3 among which the oxidation of 4-hydroxyquinolin-2-ones3a,b and intramolecular cyclization of N-acylanthranilates1d,f,3c are predominant, multiple synthetic steps and/or complex substrates limited the application of these methods. Thus the development of an efficient strategy for the synthesis of quinoline-2,4-diones is of great interest.
Nitriles are widely used in organic synthesis and the chemical industry. The cyano group can be easily transformed into various functional groups such as amines, carboxylic acids and ketones.4 Addition to nitriles is an important reaction of carbon–carbon and carbon–heteroatom bonds formation. This addition reaction proceeded mainly through the insertions of cyano groups from lithium reagents,5 Grignard reagents6 and transition-metal complexes.7 Generally, radical additions to the polar cyano group are unfavorable processes because highly unstable iminyl radicals are generated.8 However, the radical reaction might be successful if the unstable iminyl radical are effectively trapped. Many reports showed that this radical addition was carried out by transition-metal complexes such as Sm,9 Ti,10 and Mn.11 On the other hand, alcohols are a readily available fundamental bulk chemicals and widely used as starting materials and solvents in organic synthesis.12 Recently, we have launched the Ag-catalyzed radical decarboxylative addition/cyclization of α-keto acids with o-cyanoarylacrylamides employing (NH4)2S2O8 as the oxidant, where the cyclization was accomplished by an intramolecular addition of the carbon radical to the cyano group. Furthermore, a similar metal free oxidative radical cascade of aldehydes with o-cyanoarylacrylamides was also developed. This transformation provides an efficient and straightforward access to carbonyl substituted quinoline-2,4(1H,3H)-diones.13a In addition, Li and co-workers reported a new oxidative tandem route to carbonyl-containing oxindoles by iron-catalyzed oxidative 1,2-carboacylation of activated alkenes with alcohols.14 Inspired by this work, we postulated that the above radical addition/cyclization might also be achieved if α-keto acids or aldehydes was replaced by alcohols. As part of our continuing interest in radical cyclizations,13 herein, we report an iron-catalyzed addition/cyclization cascade of activated alkenes with alcohols for the synthesis of valuable carbonyl substituted quinoline-2,4(1H,3H)-diones (Scheme 1).
Initially, the reaction of anthranilonitrile-derived N-(2-cyanophenyl)-N-methylmethacrylamide (1a) with benzyl alcohol (2b) was used as the model to screen for experimental conditions. When acrylamide 1a was treated with 5.0 equiv. of benzyl alcohol in the presence of 10 mol% of FeCl3 and 3.0 equiv. of tert-butyl hydroperoxide (TBHP, 70% solution in H2O) in EtOAc (3.0 mL) at 120 °C, the reaction afforded the desired quinoline-2,4-dione 3a in 31% yield (Table 1, entry 1). Encouraged by this result, different Fe catalysts such as FeBr3, Fe(acac)3, FeCl2, FeBr2 and Fe(OAc)2 were examined; FeCl2 gave the highest yield (Table 1, entries 1–6). The effect of solvents was investigated, and EtOAc was shown to be the optimal solvent for this reaction (Table 1, entries 6–11). This transformation could occur in the presence of other oxidants such as benzoyl peroxide (BPO) and tert-butyl peroxybenzoate (TBPB) (Table 1, entries 12 and 13). No target product was observed when di-tert-butyl peroxide (DTBP), dicumyl peroxide (DCP) and K2S2O8 were used (Table 1, entries 14–16). Subsequently, the effect of substrate concentration was investigated. When the 1a concentration was increased to 0.2 M, the yield of quinoline-2,4-dione improved to 76% yield (Table 1, entry 6 and entry 17). The amount of FeCl2 was also evaluated, and the results demonstrate that a loading of 5 mol% FeCl2 gave the best result, led to the desired product in 78% yield (Table 1, entries 17–19). Decreasing the temperature negatively affected the reaction (Table 1, entry 20). It is noteworthy that the reaction cannot take place without TBHP (Table 1, entry 21), whereas, a lower yield (15%) was obtained in the absence of FeCl2 (Table 1, entry 22).
| Entry | Catalyst (mol%) | Oxidant | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (1.5 mmol), catalyst, and oxidant (3.0 equiv.) in solvent (3.0 mL) at 120 °C for 12 h.b Isolated yield.c 1.5 mL solvent.d 100 °C. | ||||
| 1 | FeCl3 (10) | TBHP | EtOAc | 31 |
| 2 | FeBr3 (10) | TBHP | EtOAc | 27 |
| 3 | Fe(acac)3 (10) | TBHP | EtOAc | 55 |
| 4 | Fe(OAc)2 (10) | TBHP | EtOAc | 12 |
| 5 | FeBr2 (10) | TBHP | EtOAc | 61 |
| 6 | FeCl2 (10) | TBHP | EtOAc | 70 |
| 7 | FeCl2 (10) | TBHP | THF | 25 |
| 8 | FeCl2 (10) | TBHP | Acetone | Trace |
| 9 | FeCl2 (10) | TBHP | CH3CN | Trace |
| 10 | FeCl2 (10) | TBHP | Dioxane | Trace |
| 11 | FeCl2 (10) | TBHP | Toluene | 50 |
| 12 | FeCl2 (10) | BPO | EtOAc | 27 |
| 13 | FeCl2 (10) | TBPB | EtOAc | 38 |
| 14 | FeCl2 (10) | DTBP | EtOAc | 0 |
| 15 | FeCl2 (10) | DCP | EtOAc | 0 |
| 16 | FeCl2 (10) | K2S2O8 | EtOAc | 0 |
| 17c | FeCl2 (10) | TBHP | EtOAc | 76 |
| 18c | FeCl2 (5) | TBHP | EtOAc | 78 |
| 19c | FeCl2 (3) | TBHP | EtOAc | 60 |
| 20c,d | FeCl2 (5) | TBHP | EtOAc | 48 |
| 21 | FeCl2 (5) | — | EtOAc | 0 |
| 22 | — | TBHP | EtOAc | 15 |
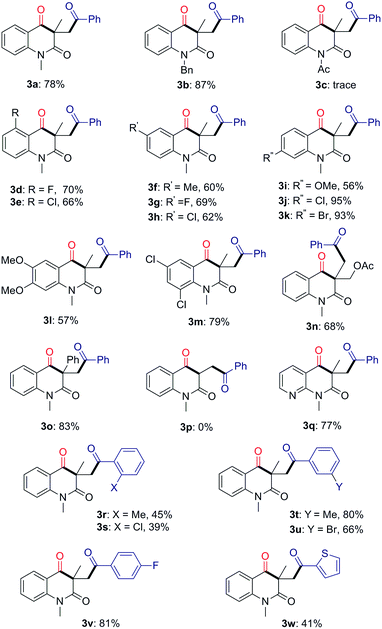
With the optimized conditions in hand, we set out to explore the substrate scope for this radical cascade and representative results were summarized in Table 2. Initially, o-cyanoarylacrylamides were examined. The o-cyanoarylacrylamides bearing electron-donating protecting groups such as methyl and benzyl on the nitrogen atom were found to be suitable substrates for the reaction, gave the desired products in good yields (3a, 3b). However, the reaction of acetylated substrate failed (3c). A substituent on the phenyl ring has no significant influence on the reaction (3d–3k). The substrates are good for this transformation, affording the quinoline-2,4-diones in moderate to excellent yields. Notably, the substrates bearing Cl and Br at para position of the phenyl ring produced 3j and 3k in 95% and 93% yields, respectively. Multisubstituted amides were also reacted well with benzyl alcohol, affording the desired products 3l and 3m in moderate yields. The substrates with different substituents on olefin were next explored. When R2 was replaced by –CH2OAc or –Ph, the reaction proceeded smoothly in moderate to good yields as well (3n, 3o). However, no reaction occurred in the case of mono-substituent olefin (R2 = H, 3p). It was noted that heterocyclic substrate pyridineacrylamide also reacted smoothly to give the desired product 3q in 77% yield. The scope of alcohols was also examined. The results demonstrated that both electron-donating and electron-withdrawing groups, such as Me, MeO, F, Cl and Br groups, on the aromatic ring of the benzyl moiety were well-tolerated (3r–3v). However, steric effect on the aromatic ring was also observed in this transformation. The ortho-substituted benzyl alcohols exhibited lower reactivity, and produced relatively lower yields (3r, 3s). It is noteworthy that heteroaryl methanol, 2-thiophenemethanol, was compatible with the optimal conditions, and the product 3w was obtained in 41% yield. However, alkyl alcohols such as ethanol, cyclohexanemethanol and 3-phenyl-1-propanol were inert under these conditions.
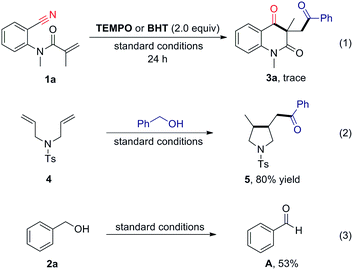
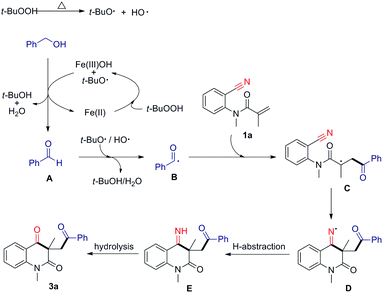
A radical pathway was firstly considered to be involved in this cascade, and several control experiments were performed (Scheme 2). This reaction was found to be remarkably suppressed by 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and butylated hydroxytoluene (BHT), and only trace amounts of the desired products were detected, even after longer reaction times. Moreover, cyclized product 5 was obtained in 80% yield when diene 4 was reacted with benzyl alcohol under the standard conditions. These results suggest that the transformation may involve a radical pathway. In addition, benzyl alcohol could be readily converted into benzaldehyde in the presence of FeCl2 and TBHP. It indicate that aldehyde is key intermediate in this transformation.
On the basis of the above experimental results and the literature reports,13a,15 a possible mechanism is proposed in Scheme 3. First, the low valent iron species donates an electron to TBHP to generate Fe(III) species and the alkyloxy radical.15a Oxidation of alcohol by Fe(III) species and alkyloxy radical to give the aldehyde A. Meanwhile, the low valent iron species was regenerated.15b Under heating conditions, TBHP is readily split into alkyloxy and hydroxy radicals. Subsequently, aldehydic hydrogen is abstracted by alkyloxy and/or hydroxy radicals to generate the acyl radical B.15c The radical addition of A to alkene 1a gives the alkyl radical C, followed by addition to the nitrile affords imine radical D, which then undergoes H-abstraction to give the imine E. Finally, imine is hydrolyzed to provide the desired product 3a.
Conclusions
In conclusion, we have developed an iron-catalyzed radical addition/cyclization cascade of activated alkenes with alcohols. The process exhibits wide substrate scope, good functional group tolerance and utilizing readily available reagents, thus providing a convenient and efficient pathway for the synthesis of carbonyl substituted quinoline-2,4(1H,3H)-diones.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21402071) and Kunming University of Science and Technology (KKSY201426046).Notes and references
- (a) S. Kitamura, K. Hashizume, T. Iida, E. Miyashita, K. Shirahata and H. Kase, J. Antibiot., 1986, 39, 1160 CrossRef CAS PubMed; (b) R. Laschober and W. Stadlbauer, Liebigs Ann. Chem., 1990, 1083 CrossRef CAS; (c) J. L. McCormick, T. C. McKee, J. H. Cardellina and M. R. Boyd, J. Nat. Prod., 1996, 59, 469 CrossRef CAS PubMed; (d) C. M. Seong, W. K. Park, C. M. Park, J. Y. Kong and N. S. Park, Bioorg. Med. Chem. Lett., 2008, 18, 738 CrossRef CAS PubMed; (e) N. Ahmed, K. G. Brahmbhatt, S. Sabde, D. Mitra, I. P. Singh and K. K. Bhutani, Bioorg. Med. Chem., 2010, 18, 2872 CrossRef CAS PubMed; (f) Y.-X. Liu, H.-P. Zhao, Z.-W. Wang, Y.-H. Li, H.-B. Song, H. Riches, D. Beattie, Y.-C. Gu and Q.-M. Wang, Mol. Diversity, 2013, 17, 701 CrossRef CAS PubMed; (g) S. Han, F.-F. Zhang, H.-Y. Qian, L.-L. Chen, J.-B. Pu, X. Xie and J.-Z. Chen, J. Med. Chem., 2015, 58, 5751 CrossRef CAS PubMed.
- (a) M. A. Chauncey and M. F. Grundon, Synthesis, 1990, 1005 CrossRef CAS; (b) A. Klásek, A. Lyčka, M. Holčapek and I. Hoza, Tetrahedron, 2004, 60, 9953 CrossRef; (c) A. Klásek, V. Mrkvička, A. Pevec and J. Košmrlj, J. Org. Chem., 2004, 69, 5646 CrossRef PubMed; (d) E. J. Jung, B. H. Park and Y. R. Lee, Green Chem., 2010, 12, 2003 RSC.
- (a) R. Kumabe and H. Nishino, Tetrahedron Lett., 2004, 45, 703 CrossRef CAS; (b) S. Kafka, K. Proisl, V. Kasparkova, D. Urankar, R. Kimmel and J. Kosmrlj, Tetrahedron, 2013, 69, 10826 CrossRef CAS; (c) W. S. Hamama, A. El-Din, E. Hassanien and H. H. Zoorob, Synth. Commun., 2014, 44, 1833 CrossRef CAS; (d) S. A. Antolak, Z.-K. Yao, G. M. Richoux, C. Slebodnick and P. R. Carlier, Org. Lett., 2014, 16, 5204 CrossRef CAS PubMed; (e) A. L. Zografos, C. A. Mitsos and O. Igglessi-Markopoulou, Org. Lett., 1999, 1, 1953 CrossRef CAS; (f) Y. S. Shin, S. J. Song, S. Kang, H. S. Hwang, Y.-S. Jung and C.-H. Kim, J. Appl. Toxicol., 2014, 34, 191 CrossRef CAS PubMed.
- (a) A. J. Fatiadi, in Preparation and Synthetic Applications of Cyano Compounds, ed. S. Patai and Z. Rappaport, Wiley, New York, 1983 Search PubMed; (b) T. Wang and N. Jiao, Acc. Chem. Res., 2014, 47, 1137 CrossRef CAS PubMed.
- M. S. Malamas, J. Erdei, I. Gunawan, K. Barnes, M. Johnson, Y. Hui, J. Turner, Y. Hu, E. Wagner, K. Fan, A. Olland, J. Bard and A. J. Robichaud, J. Med. Chem., 2009, 52, 6314 CrossRef CAS PubMed.
- (a) W. Clegg, R. P. Davies, L. Dunbar, N. Feeder, S. T. Liddle, R. E. Mulvey, R. Snaith and A. E. H. Wheatley, Chem. Commun., 1999, 1401 RSC; (b) S. Jie, S. Zhang and W.-H. Sun, Eur. J. Inorg. Chem., 2007, 5584 CrossRef CAS.
- For selected papers, see: (a) C. Zhou and R. C. Larock, J. Am. Chem. Soc., 2004, 126, 2302 CrossRef CAS PubMed; (b) B. Zhao and X. Lu, Org. Lett., 2006, 8, 5987 CrossRef CAS PubMed; (c) J. Lindh, P. J. R. Sjöberg and M. Larhed, Angew. Chem., Int. Ed., 2010, 49, 7733 CrossRef CAS PubMed; (d) J. Liu, X. Zhou, H. Rao, F. Xiao, C.-J. Li and G.-J. Deng, Chem.–Eur. J., 2011, 17, 7996 CrossRef CAS PubMed; (e) T.-S. Jiang and G.-W. Wang, Org. Lett., 2013, 15, 788 CrossRef CAS PubMed; (f) Y. Ma, J. You and F. Song, Chem.–Eur. J., 2013, 19, 1189 CrossRef CAS PubMed; (g) G. Xia, X. Han and X. Lu, Org. Lett., 2014, 16, 2058 CrossRef CAS PubMed; (h) T. Miura, H. Nakazawa and M. Murakami, Chem. Commun., 2005, 2855 RSC; (i) T. Miura and M. Murakami, Org. Lett., 2005, 7, 3339 CrossRef CAS PubMed; (j) G. C. Tsui, Q. Glenadel, C. Lau and M. Lautens, Org. Lett., 2011, 13, 208 CrossRef CAS PubMed; (k) J.-C. Hsieh, Y.-C. Chen, A.-Y. Cheng and H.-C. Tseng, Org. Lett., 2012, 14, 1282 CrossRef CAS PubMed.
- (a) D. P. Curran, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Flemming, Pergamon Press, Oxford, UK, 1991, vol. IV Search PubMed; (b) A. L. J. Beckwith and K. D. Raner, J. Org. Chem., 1992, 57, 4954 CrossRef CAS; (c) S. Kim, Adv. Synth. Catal., 2004, 346, 19 CrossRef CAS.
- (a) G. A. Kraus and J. O. Sy, J. Org. Chem., 1989, 54, 77 CrossRef CAS; (b) G. A. Molander and C. N. Wolfe, J. Org. Chem., 1998, 63, 9031 CrossRef CAS.
- (a) Y. Yamamoto, D. Matsumi, R. Hattori and K. Itoh, J. Org. Chem., 1999, 64, 3224 CrossRef CAS PubMed; (b) J. Streuff, M. Feurer, P. Bichovski, G. Frey and U. Gellrich, Angew. Chem., Int. Ed., 2012, 51, 8661 CrossRef CAS PubMed.
- B. B. Snider and B. O. Buckman, J. Org. Chem., 1992, 57, 322 CrossRef CAS.
- (a) M. Hudlicky, Oxidations in Organic Chemistry, American Chemical Society, Washington, D. C, 1990 Search PubMed; (b) R. A. Sheldon and J. K. Kochi, Metal-Catalysed Oxidations of Organic Compounds, Academic Press, New York, 1981 Search PubMed; (c) T. Mallat and A. Baiker, Chem. Rev., 2004, 104, 3037 CrossRef CAS PubMed; (d) R. Uma, C. Crévisy and R. Grée, Chem. Rev., 2003, 103, 27 CrossRef CAS PubMed.
- (a) S.-S. Wang, H. Fu, Y. Shen, M. Sun and Y.-M. Li, J. Org. Chem., 2016, 81, 2920 CrossRef CAS PubMed; (b) Y.-M. Li, S.-S. Wang, F.-C. Yu, Y. Shen and K.-J. Chang, Org. Biomol. Chem., 2015, 13, 5376 RSC; (c) Y.-M. Li, Y. Shen, K.-J. Chang and S.-D. Yang, Tetrahedron Lett., 2014, 55, 2119 CrossRef CAS; (d) Y.-M. Li, Y. Shen, K.-J. Chang and S.-D. Yang, Tetrahedron, 2014, 70, 1991 CrossRef CAS; (e) Y.-M. Li, X.-H. Wei, X.-A. Li and S.-D. Yang, Chem. Commun., 2013, 49, 11701 RSC; (f) Y.-M. Li, M. Sun, H.-L. Wang, Q.-P. Tian and S.-D. Yang, Angew. Chem., Int. Ed., 2013, 52, 3972 CrossRef CAS PubMed.
- X.-H. Ouyang, R.-J. Song and J.-H. Li, Eur. J. Org. Chem., 2014, 3395 CrossRef CAS.
- (a) W. Liu, Y. Li, K. Liu and Z. Li, J. Am. Chem. Soc., 2011, 133, 10756 CrossRef CAS PubMed; (b) J. M. Hoover, B. L. Ryland and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 2357 CrossRef CAS PubMed; (c) M.-B. Zhou, R.-J. Song, X.-H. Ouyang, Y. Liu, W.-T. Wei, G.-B. Deng and J.-H. Li, Chem. Sci., 2013, 4, 2690 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, copies of the 1H and 13C NMR spectra. See DOI: 10.1039/c6ra08976c |
| This journal is © The Royal Society of Chemistry 2016 |