Infrared ray assisted microwave synthesis: a convenient method for large-scale production of graphitic carbon nitride with outstanding nitrogen photofixation ability†
Shijun Li,
Xin Chen,
Shaozheng Hu*,
Qiang Li,
Jin Bai and
Fei Wang
College of Chemistry, Chemical Engineering, and Environmental Engineering, Liaoning Shihua University, Fushun 113001, China. E-mail: hushaozhenglnpu@163.com
First published on 5th May 2016
Abstract
Nitrogen fixation is the second most important chemical process in nature next to photosynthesis. Both the energy consumption and raw material costs are high for the conventional artificial nitrogen fixation technology, the Haber–Bosch process. Here, we report a convenient infrared ray assisted microwave method for synthesizing graphitic carbon nitride (g-C3N4) with outstanding nitrogen photofixation ability under visible light. XRD, N2 adsorption, UV-vis, SEM, TEM, TPD, EPR, PL and photocurrent measurements were used to characterize the prepared catalysts. The results indicate that microwave treatment can form many irregular pores in the as-prepared g-C3N4, which cause an increase in the surface area and promote the separation rate of electrons and holes. More importantly, microwave treatment causes the formation of many nitrogen vacancies in the as-prepared g-C3N4. These nitrogen vacancies not only serve as active sites to adsorb and activate N2 molecules but also promote interfacial charge transfer from catalysts to N2 molecules, thus significantly improving the nitrogen photofixation ability. The higher nitrogen vacancies concentration of g-C3N4 prepared by infrared ray assisted microwave treatment causes more chemical adsorption sites, leading to a higher nitrogen photofixation performance. Moreover, the present process is a convenient method for large-scale production of g-C3N4 which is significantly important for practical applications.
Introduction
Nitrogen is an essential building element of plants and animals. Because the bond energy of the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond is too high, molecular nitrogen cannot be utilized by the organism directly. Most plants can take up nitrogen in the form of ammonium ion (NH4+). Thus nitrogen fixation is the second most important chemical process in nature next to photosynthesis. However, the natural nitrogen fixation does not satisfy the increased demand of modern agriculture.1,2 Artificial nitrogen fixation is carried out through the Haber–Bosch process, in which hydrogen gas reacts with nitrogen gas to yield ammonia in the presence of catalysts under high pressure and temperature. Both the energy consumption and raw material costs are high for this process. Therefore, artificial nitrogen fixation under milder conditions is of considerable significance from the perspectives of cost and environmental protection.
N triple bond is too high, molecular nitrogen cannot be utilized by the organism directly. Most plants can take up nitrogen in the form of ammonium ion (NH4+). Thus nitrogen fixation is the second most important chemical process in nature next to photosynthesis. However, the natural nitrogen fixation does not satisfy the increased demand of modern agriculture.1,2 Artificial nitrogen fixation is carried out through the Haber–Bosch process, in which hydrogen gas reacts with nitrogen gas to yield ammonia in the presence of catalysts under high pressure and temperature. Both the energy consumption and raw material costs are high for this process. Therefore, artificial nitrogen fixation under milder conditions is of considerable significance from the perspectives of cost and environmental protection.
In 1977, Schrauzer et al. first reported that N2 can be reduced to NH3 over Fe-doped TiO2 under UV light.3 Since then, because of the advantages such as green cleaning, mild conditions, low power consumption and low cost, photocatalytic nitrogen fixation technology is considered to be the best alternative to the Haber–Bosch process. Many Ti-based metal oxides and composite catalysts have been reported successively.4–8 However, because of the poor visible light absorption caused by the wide band gap energy, the nitrogen fixation ability of the Ti-based metal oxides and composite catalysts is still low under visible light. Thus, in recent years, many novel nitrogen-photofixation systems are reported successively.9–12
Recently, graphitic carbon nitride (g-C3N4) has been widely applied in a variety of fields, including photocatalysis,13–15 fuel cells,16 organic synthesis17 and gas storage.18,19 In general, g-C3N4 is synthesized by calcination raw materials at 500–550 °C for several hours. During this process, because of the temperature gradient, the surface temperature of the raw materials is much higher than that in the bulk. This results in that the polycondensation occur on the surface of raw materials with producing harmful gases. At the same time, the polycondensation has not yet occurred in the bulk of raw materials because of the not satisfied temperature. This leads to high energy consumption, large emission of harmful gas and low catalyst yield.
In 2014, Yuan et al. reported a rapid synthetic strategy named “microwave-assisted heating synthesis”.20 The microwave-assisted processes involve energy transfer from microwave to the microwave-absorber materials which induces strong heating in minutes. When the microwave energy is absorbed by the raw material, the molecules are orderly arrangement in the electromagnetic field of the microwave. Then the high frequency reciprocating motion occurs inside the molecules of raw materials, causes the frequent collisions between molecules, leading to the generation of a lot of frictional heat. Under this heating method, the raw material is rapidly heated without the presence of temperature gradient. For microwave method, the microwave absorbing material is required to heat conduction. However, the heat transfer performance between solids is uneven and unsatisfactory.
In recent years, infrared heating has been widely applied in many fields, such as material synthesis, food hygiene, medical insurance and solar battery. Because the frequency of infrared ray and molecular vibration is close, resonance effects will greatly increase the amplitude of molecular vibration, thus improve the molecular kinetic energy. More importantly, this method does not need the heat transfer medium. Thus, in this work, infrared ray assisted microwave method is used to prepare g-C3N4 catalysts. Characterization results indicate that many nitrogen vacancies are formed in the as-prepared g-C3N4 catalysts. Li et al. discovered that the introduction of oxygen vacancies in BiOBr photocatalyst could activate N2 and promote interfacial electron transfer, thus significantly improving the nitrogen photofixation ability.21 We hypothesize that nitrogen vacancies may be more effective than oxygen vacancies for nitrogen photofixation because nitrogen vacancies have the same shape and size as the nitrogen atoms in N2 molecules. N2 can be adsorbed and activated more easily on nitrogen vacancies. Thus the nitrogen photofixation performance under visible light was tested to evaluate the performance of the as-prepared catalysts. From the standpoint of energy saving and cost reduction, NH4+ production rate per kilowatt of energy consumption (r(NH4+)/kW) and NH4+ production rate per gram raw material (r(NH4+)/gmaterial) are used to compare three catalysts prepared by calcination, microwave and infrared ray assisted microwave method.
Experimental
Preparation and characterization
In a typical process, 6 g of thiourea was grounded for 30 min in a mortar and then transferred to an alumina crucible (25 mL). This crucible was then put into another alumina crucible (200 mL), buried with the CuO powder (microwave absorbing material), and treated by infrared ray assisted microwave for 20–40 min in a normal microwave oven (G70D20CN1P-D2, Galanz). The obtained catalysts were denoted as IM-CN(x), where x stands for the treated time of infrared ray assisted microwave (min). When only microwave (15–30 min) was used to synthesize g-C3N4 following the same procedure mentioned above, the obtained catalysts were denoted as M-CN(x), where x stands for treated time of microwave (min). For comparison, thiourea was heated at 520 °C for 2 h at the rate of 5 °C min−1, and denoted as CN520.The XRD patterns of the prepared samples were recorded on a Rigaku D/max-2400 instrument using Cu-Kα radiation (λ = 1.54 Å). The scan rate, step size, voltage and current were 0.05° min−1, 0.01°, 40 kV and 30 mA, respectively. UV-vis spectroscopy was carried out on a JASCO V-550 model UV-vis spectrophotometer using BaSO4 as the reflectance sample. The morphologies of prepared catalyst were observed by using a scanning electron microscope (SEM, JSM 5600LV, JEOL Ltd.). TEM images were taken on a Philips Tecnai G220 model microscope. Nitrogen adsorption was measured at −196 °C on a Micromeritics 2010 analyser. All the samples were degassed at 393 K prior to the measurement. The BET surface area (SBET) was calculated based on the adsorption isotherm. Elemental analysis was performed with a vario EL cube from Elementar Analysensysteme GmbH. Electron paramagnetic resonance (EPR) spectrum was monitored using a digital X-band spectrometer (EMX-220, Bruker, USA) equipped with a Bruker ER 4121VT temperature controller within the temperature range 113–273 K. The XPS measurements were performed on a Thermo Escalab 250 XPS system with Al Kα radiation as the excitation source. The binding energies were calibrated by referencing the C 1s peak (284.6 eV) to reduce the sample charge effect. Temperature Programmed Desorption (TPD) studies were performed using a CHEMBET-3000 (Quantachrome, U.S.A.) instrument in the temperature range of 313 to 1073 K. The photoluminescence (PL) spectra were measured at room temperature with a fluorospectrophotometer (FP-6300) using a Xe lamp as the excitation source. The Mott–Schottky plots were obtained using an electrochemical analyzer (LK2006A, Lanlike) using a three-electrode cell. The photocurrents were measured using an electrochemical analyzer (CHI 618C Instruments) equipped with a rectangular-shaped quartz reactor (20 × 40 × 50 mm) using a standard three-electrode system. The prepared sample film was used as the working electrode, a Pt flake was used as the counter electrode, and Ag/AgCl was used as the reference electrode. A 500 W Xe lamp was used to irradiate the working electrode from the back side. The light intensity on the working electrode was 120 mW cm−2. In addition, a mechanical shutter was used to minimize the exposure of the sample to light. A 1.0 M Na2SO4 solution was used as the electrolyte. The applied potential was 0.00 V vs. Ag/AgCl. All the measurements were performed at room temperature (298 K).
Photocatalytic reaction
The nitrogen photofixation property was evaluated according to previous literature.8 The nitrogen photofixation experiments were performed in a double-walled quartz reactor in air. For these experiments, 0.2 g of photocatalyst was added to a 500 mL 0.789 g L−1 ethanol as a hole scavenger.8 The suspension was dispersed using an ultrasonicator for 10 min. During the photoreaction under visible light irradiation, the suspension was exposed to a 250 W high-pressure sodium lamp with main emission in the range of 400 to 800 nm, and N2 was bubbled at 100 mL min−1 through the solution. The UV light portion of the sodium lamp was filtered by a 0.5 M NaNO2 solution. All runs were conducted at ambient pressure and 30 °C. At given time intervals, 5 mL aliquots of the suspension were collected and immediately centrifuged to separate the liquid samples from the solid catalyst. The concentration of ammonia was measured using the Nessler's reagent spectrophotometry method (JB7478-87) with a UV-2450 spectrophotometer (Shimadzu, Japan).8,21RhB was selected as the model compound to evaluate the photocatalytic performance of the prepared g-C3N4 in aqueous solution under visible light irradiation. For this purpose, 0.05 g of catalyst was dispersed in 200 mL of an aqueous solution of RhB (10 ppm) in an ultrasonicator for 10 min. The suspension was transferred into a lab-designed glass reactor and stirred for 30 min in darkness to achieve adsorption equilibrium. The photoreaction under visible light was performed using the same light source as that used for nitrogen photofixation. The concentrations of RhB before and after the reaction were measured using a UV-vis spectrophotometer at a wavelength of 550 nm.
Results and discussion
Fig. 1 shows the nitrogen photofixation performance over the as-prepared catalysts under visible light. The results of the control experiment indicate that the NH4+ production rate can be ignored in the absence of irradiation, N2 or photocatalyst, indicating that nitrogen photofixation occurs via a photocatalytic process (Fig. S1†). In Fig. 1a, CN520 shows the NH4+ production rate (r(NH4+)) of 1.02 mg L−1 h−1 gcat−1. For M-CN(20), r(NH4+) increases to 2.0 mg L−1 h−1 gcat−1. It is noted that the r(NH4+) of IM-CN(30) further increases to 5.1 mg L−1 h−1 gcat−1, which is 5-fold and 2.5-fold higher than those of CN520 and M-CN(20), respectively. The Fig. 1b shows the photocatalytic stabilities of IM-CN(30). No obvious decrease in nitrogen photofixation ability is observed after 20 h, hinting its good stability. Fig. 1c and d shows the NH4+ production rate per kilowatt of energy consumption (r(NH4+)/kW) and NH4+ production rate per gram raw material (r(NH4+)/gmaterial) of as-prepared catalysts. The r(NH4+)/kW and r(NH4+)/gmaterial for CN520 are 0.2 and 0.32 mg L−1 h−1 gcat−1. For M-CN(20), these values increase to 5.5 and 0.6 mg L−1 h−1 gcat−1. For IM-CN(30), these values further increase to 12.5 and 4.0 mg L−1 h−1 gcat−1. These results hints that the infrared ray assisted microwave treatment is an effective method to further improve the nitrogen photofixation performance of catalysts from the standpoint of energy saving and cost reduction. In addition, the addition of AgNO3 as electron scavenger sharply suppresses the nitrogen photofixation ability of IM-CN(30) (Fig. S2a†), indicating the main active species are the photogenerated electrons. Using aprotic solvents (DMF and DMSO) instead of water shows that no NH4+ is generated, confirming the necessity of H2O as the proton source for the nitrogen photofixation (Fig. S2b†). H2 production is a possible competitive reaction. Thus the photocatalytic H2 production experiment is performed according to previous work.22 The result shown in Table S1† indicates that the H2 production ability of as-prepared catalysts is negligible, which is probably due to the absence of a proper co-catalyst.The XRD patterns of as-prepared catalysts under various conditions are shown in Fig. 2. CN520 shows the typical characteristic peaks of g-C3N4 located at 13.1 and 27.5°. The characteristic peaks of g-C3N4 appear after 15 min (20 min) microwave (infrared ray assisted microwave) treatment. This indicates the graphitic carbon nitride can be formed in a very short time by microwave method. However, some impurity peaks still exist after this short treated time. Further extending the microwave time leads to the formation of g-C3N4 materials with perfect crystal structure. 30 min is needed for forming the g-C3N4 structure under infrared ray assisted microwave treatment, which is longer than that of single microwave treatment (20 min). This is because the input power of single microwave (1 kW h−1) is higher than that of infrared ray assisted microwave treatment (0.8 kW h−1). It is noted that, compared with CN520, a 0.3° shift to lower 2θ value is observed for both microwave and infrared ray assisted microwave treated g-C3N4 materials. This is probably due to that microwave treatment causes some crystal lattice defects in g-C3N4. The C/N ratio for CN520 is 0.74 obtained by elemental analysis, close to the theoretical values. For M-CN(20) and IM-CN(30), the C/N ratio is 0.78 and 0.82, respectively. Combine with the XRD results, it is deduced that the crystal lattice defects in g-C3N4 should be the nitrogen vacancies. The higher C/N ratio for IM-CN(30) causes the higher nitrogen vacancies concentration compared with M-CN(20). Why infrared ray irradiation can influence the structure of g-C3N4 material is not clear till now. We propose that the infrared ray assisted heating method could change the polycondensation degree of raw material, leading to the formation of more nitrogen vacancies. Besides, no sulfur element is found in the catalysts, which is consistent with previous result.23
 | ||
| Fig. 2 XRD patterns of as-prepared catalysts under microwave (a) and infrared ray assisted microwave (b) treatment. | ||
The morphologies of the representative samples were examined by SEM analysis (Fig. 3). The result in Fig. 3a indicates that CN520 displays layer structure that is similar to the analogue graphite. For M-CN(20) and IM-CN(30) (Fig. 3b and c), layer structures are also observed, accompanying with the formation of many irregular pores. No obvious morphological difference between M-CN(20) and IM-CN(30) is observed. It is deduced from the SEM results that the microwave treatment but not infrared radiation has a significant influence on the morphology of g-C3N4, which most likely affects its photocatalytic performance. The typical HRTEM image of IM-CN(30) exhibits the clear lattice fringe (Fig. 3d). The measured lattice spacings are 0.330, very close to the (0 0 2) crystal face of graphitic carbon nitride.24–26 This confirms that the graphitic carbon nitride has been successfully synthesized by this infrared ray assisted microwave method.
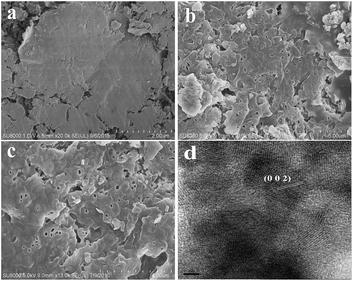 | ||
| Fig. 3 SEM images of as-prepared CN520 (a), M-CN(20) (b), IM-CN(30) (c) and HRTEM image of IM-CN(30) (d). | ||
Generally, a catalyst with high specific surface area (SBET) is significant to the enhancement of catalytic performance.27 The nitrogen adsorption and desorption isotherms of CN520, M-CN(20) and IM-CN(30) were measured (Fig. S3†). The isotherms of as-prepared catalysts are of classical type IV, suggesting the presence of mesopores (2–50 nm). Because of the agglomeration, CN520 shows a low SBET of 9.4 m2 g−1. The SBET of M-CN(20) and IM-CN(30) are 45.6 and 47.2 m2 g−1, much higher than that of CN520. This is probably due to the formation of many pores after microwave treatment which is consistent with the SEM results. In order to eliminate the influence of surface area on the N2 photofixation ability, the g-C3N4 catalyst with high surface area was prepared according to previous work,28 and denoted as MCN540. The C/N ratio for MCN540 is 0.74 obtained by ICP. The N2 adsorption result shows that the surface area of MCN540 is 44.6 m2 g−1, close to that of M-CN(20) and IM-CN(30). However, the NH4+ production rate of MCN540 is only 1.44 mg L−1 h−1 gcat−1, much lower than that of M-CN(20) and IM-CN(30). This hints that the increased surface area of microwave treated g-C3N4 catalysts is not the main factor which improves the N2 photofixation ability.
Fig. 4a compares the UV-vis spectra of the as-prepared CN520, M-CN(20) and IM-CN(30). All the catalysts show typical semiconductor absorption. The band gap energy calculated based on the method of Oregan and Gratzel indicates that the value for CN520 is 2.75 eV.29 M-CN(20) has an absorption edge at ∼465 nm, corresponding to a band gap of 2.67 eV. For IM-CN(30), the absorption edge further shifts to 472 nm, corresponding to a band gap of 2.62 eV. Considering the absence of sulfur element in the catalysts, this decreased band gap energy of microwave treated g-C3N4 catalysts is probably due to the presence of nitrogen vacancies, leading to changes in the electronic structures and optical properties. It is noted that the absorption tail in the whole visible light region is observed in the spectra of M-CN(20) and IM-CN(30) but not CN520. This absorption tail should be due to the formation of nitrogen vacancies in microwave treated g-C3N4 catalysts.30 The CB potential of as prepared CN520, M-CN(20) and IM-CN(30) using Mott–Schottky plots was measured and shown in Fig. 4b. The ECB is −1.16, −1.12 and −1.1 V for CN520, M-CN(20) and IM-CN(30), respectively. Combine with the band gap results obtained from UV-vis spectra, the EVB of CN520, M-CN(20) and IM-CN(30) located at +1.59, +1.55 and +1.52 V, respectively. The photogenerated electrons on CN520 should have the stronger reduction capability than that of M-CN(20) and IM-CN(30). Thus it is deduced that the influence of nitrogen vacancies on the band structure of as-prepared carbon nitride is not the main factor to enhance its photocatalytic nitrogen fixation activity.
 | ||
| Fig. 4 UV-vis spectra (a), Mott–Schottky plots (b), EPR spectra (c) of as-prepared catalysts and the mass spectra of the indophenol prepared from different atmosphere (d). | ||
EPR can provide direct information on monitoring various behaviors of native defects, such as oxygen and nitrogen vacancies.31,32 As shown in Fig. 4c, CN520 shows no peaks, suggesting that no localized unpaired electrons present in the CN520. However, for IM-CN(30) and M-CN(20), a resonance signal at g = 2.0031 is observed, which confirms the presence of nitrogen vacancies. In order to further investigate the nitrogen source of NH4+, the N2 photofixation ability of IM-CN(30) under 15N isotope-labeled N2 (purity > 98%) was performed. The produced 15NH4+ reacts with phenolic and hypochlorite to form 15N labeled indophenol, which was analyzed by LC-MS. A strong 15N labeled indophenol anion mass spectroscopy signal presented at 199 m/z in LC-MS studies (Fig. 4d). It is note that the intensity of this signal was obviously higher than that of the 14N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15N natural abundance ratio. This observation further confirmed that N2 was the source of generated ammonium ion in this N2 photofixation process.
15N natural abundance ratio. This observation further confirmed that N2 was the source of generated ammonium ion in this N2 photofixation process.
The surface chemical compositions of the as-prepared g–C3N4–based catalysts were characterized using XPS. In Fig. 5a (N 1s region), the two contributions of CN520 located at 398.5 and 400.2 eV are assigned to the sp2-hybridized aromatic nitrogen atoms bonded to carbon atoms (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and nitrogen atoms bonded to three carbon atoms (N–C3).33 For M-CN(20) and IM-CN(30), no obvious difference in peak position is observed. However, the peak area ratio of (N–C3)/(C–N
C) and nitrogen atoms bonded to three carbon atoms (N–C3).33 For M-CN(20) and IM-CN(30), no obvious difference in peak position is observed. However, the peak area ratio of (N–C3)/(C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) decreases from 0.27 for CN520 to 0.25 for M-CN(20) and 0.24 for IM-CN(30), clearly indicating that nitrogen vacancies are primarily located at the tertiary nitrogen lattice sites. For the C 1s region (Fig. 5b), the three contributions located at 284.6, 286 and 288.4 eV for CN520 are attributed to C–C bonds, which originated from sp2 C atoms bonded to N in an aromatic ring (N–C
C) decreases from 0.27 for CN520 to 0.25 for M-CN(20) and 0.24 for IM-CN(30), clearly indicating that nitrogen vacancies are primarily located at the tertiary nitrogen lattice sites. For the C 1s region (Fig. 5b), the three contributions located at 284.6, 286 and 288.4 eV for CN520 are attributed to C–C bonds, which originated from sp2 C atoms bonded to N in an aromatic ring (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); C
N); C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N or C
N or C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, which could be attributed to defect-containing sp2-hybridized carbon atoms present in graphitic domains; and pure graphitic sites in a CN matrix.34,35 Note that, in addition to the three C 1s peaks mentioned above, a new peak at a high binding energy of 290 eV appears in M-CN(20) and IM-CN(30). This peak is attributed to the two-coordinated carbon (N–C–N) formed by the disappearance of three-coordinated nitrogen, thereby confirming the generation of nitrogen vacancies. In addition, for IM-CN(30), the area of this peak is larger than that of M-CN(20), confirming the higher nitrogen vacancies concentration in IM-CN(30).
N, which could be attributed to defect-containing sp2-hybridized carbon atoms present in graphitic domains; and pure graphitic sites in a CN matrix.34,35 Note that, in addition to the three C 1s peaks mentioned above, a new peak at a high binding energy of 290 eV appears in M-CN(20) and IM-CN(30). This peak is attributed to the two-coordinated carbon (N–C–N) formed by the disappearance of three-coordinated nitrogen, thereby confirming the generation of nitrogen vacancies. In addition, for IM-CN(30), the area of this peak is larger than that of M-CN(20), confirming the higher nitrogen vacancies concentration in IM-CN(30).
The photocatalytic performance of RhB degradation over CN520, M-CN(20) and IM-CN(30) was also evaluated under visible light (Fig. S4†). The reaction rate constant k was obtained by assuming that the reaction followed first-order kinetics (Fig. S4,† inset).36 Prior to turning the light on, the RhB adsorption ability of M-CN(20) and IM-CN(30) is improved compared with CN520, which is probably due to the larger SBET. Due to a high recombination rate for electrons and holes, CN520 exhibits a low degradation rate (∼60%) and reaction rate constant (0.009 min−1). For M-CN(20) and IM-CN(30), the degradation rates sharply improve to 85% and 94%, probably due to the increased SBET and decreased band gap energy. The rate constants for M-CN(20) and IM-CN(30) are 0.0144 and 0.0236 min−1, which is 1.6-fold and 2.6-fold greater than that of CN520 respectively. It is noted that the r(NH4+) for M-CN(20) and IM-CN(30) is 2-fold and 5-fold higher than that of CN520, which growth rate is obvious higher than that of RhB degradation performance. Thus, it is deduced that the enhanced nitrogen photofixation ability is not only due to the increased SBET and decreased band gap energy but also due to some other reasons.
Because the chemical adsorption sites are considered to be reaction centers capable of activating N2, chemisorption is an essential step in photocatalytic N2 fixation. TPD investigations were performed to understand N2 chemisorption on the surface of the as-prepared catalysts. In Fig. 6, the N2-TPD results on CN520, M-CN(20) and IM-CN(30) are compared. Two adsorbed N2 species in M-CN(20) and IM-CN(30) and only one adsorbed N2 species in CN520 are observed. The peak at ∼120 °C is related to physical adsorption. The peak at 260 °C, which is related to the strong chemisorption species of N2, is observed for M-CN(20) and IM-CN(30) but not for CN520. This result indicates that nitrogen vacancies could introduce many chemical adsorption sites on the surface of M-CN(20) and IM-CN(30). Because chemisorption is generally associated with activation, these chemical adsorption sites will activate N2 for nitrogen photofixation. Thus the higher nitrogen vacancies concentration of IM-CN(30) causes the more chemical adsorption sites, leading to the higher nitrogen photofixation performance.
It is deduced from XPS result that the nitrogen vacancies are located at the three-coordinated nitrogen. As shown in Fig. 7, there are two possible positions for nitrogen vacancies. To further confirm that N2 is activated by nitrogen vacancies, density functional theory (DFT) simulations were employed to investigate the interaction between a N2 molecule and the g-C3N4 with two nitrogen vacancies location (Fig. 7). The optimized results indicated that the adsorption energy is −166.2 kJ mol−1 for position 1, confirming the chemisorption occurs. When the N2 molecule adsorbs on the nitrogen vacancies, an σ bond between the N2 molecule and the nearest C atom is formed, confirming N2 is mainly adsorbed on the nitrogen vacancies. The N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond is prolonged from 1.107 Å to 1.242 Å, proving that nitrogen vacancies can activate N2. However, only physical adsorption with the adsorption energy of −2.4 kJ mol−1 occurs when N2 is adsorbed on position 2. The N
N bond is prolonged from 1.107 Å to 1.242 Å, proving that nitrogen vacancies can activate N2. However, only physical adsorption with the adsorption energy of −2.4 kJ mol−1 occurs when N2 is adsorbed on position 2. The N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond length is also not changed for this situation. Thus it is deduced the nitrogen vacancies is probably located at position 1.
N bond length is also not changed for this situation. Thus it is deduced the nitrogen vacancies is probably located at position 1.
In general, at a lower PL intensity, the separation rate of the photogenerated electron–hole pairs is higher.37,38 In Fig. 8a, the PL intensity follows the sequence IM-CN(30) < M-CN(20) < CN520. Compared with CN520, the higher surface areas of M-CN(20) and IM-CN(30) results in a shorter migration distance, which is favourable for charge transfer from the bulk to the surface of the g-C3N4 material and lead to a higher separation rate. Besides that, IM-CN(30) shows the higher separation rate than that of M-CN(20) though they have the comparable SBET. This is probably due to that IM-CN(30) possesses more nitrogen vacancies which could act as electron trappers to improve carrier separation. Fig. 8b compares the PL intensity of CN520, M-CN(20) and IM-CN(30) under Ar and N2 atmospheres. Under N2 atmosphere, the PL intensities of M-CN(20) and IM-CN(30) are reduced compared with that under an Ar atmosphere. This hints that nitrogen vacancies may promote photogenerated electron transfer from these catalysts to adsorbed N2, thus improve the carrier separation. The different reduction level should be related to the different concentration of nitrogen vacancies. By contrast, no difference is observed for CN520 between the PL intensities under N2 and Ar atmospheres, confirming this point of view.
 | ||
| Fig. 8 PL spectra under N2 atmospheres (a) and the comparison of PL intensity of as-prepared CN520, M-CN(20) and IM-CN(30) under Ar and N2 atmospheres (b). | ||
Fig. 9a shows the photocurrent responses of CN520, M-CN(20) and IM-CN(30) under N2 atmospheres. Because of the higher separation rate of electrons and holes, M-CN(20) and IM-CN(30) shows the higher photocurrent than that of CN520. Note that the photocurrent generated on the CN520 electrode remains unchanged with irradiation time. However, the photocurrent densities of M-CN(20) and IM-CN(30) gradually decrease at the beginning and then remain stable. This photocurrent decay is probably due to the competition between N2 and FTO glass for trapped electrons. Li and his co-workers reported that oxygen vacancies can trap electrons and promote interfacial charge transfer from BiOBr nanosheets to N2.21 We hypothesize that nitrogen vacancies have a similar effect. The photogenerated electrons that arrived at the surface of M-CN(20) and IM-CN(30) are trapped by the nitrogen vacancies, and then transferred immediately from the catalysts to the adsorbed N2, causing the photocurrent decay.
In Fig. 9b, the photocurrents of CN520 under N2 and Ar atmosphere are almost the same. Whereas, the photocurrent of IM-CN(30) does not decay under Ar atmosphere. This result confirms the fast electron transfer process from the IM-CN(30) to the adsorbed N2. In summary, the possible nitrogen photofixation process over microwave treated g-C3N4 materials is as follows. First of all, N2 molecules are chemisorbed on the nitrogen vacancies of catalyst. When catalyst is excitated by the irradiation, the formed photogenerated-electrons are trapped by the nitrogen vacancies. Those photogenerated-electrons are transferred immediately from the catalyst to the adsorbed N2. Because the bonding orbitals of N2 molecule are occupied by four electrons, this photogenerated-electron has to occupy the anti-bonding orbitals, leading to the nitrogen activation. The activated N2 molecule reacts with H+ in water to form NH3, and then it finally forms NH4+. During this process, nitrogen vacancies not only serve as active sites to adsorb and activate N2 molecule but also promote interfacial charge transfer from g-C3N4 to N2 molecules, thereby significantly improving the nitrogen photofixation ability.
In order to compare the nitrogen photofixation ability with Ti-based catalysts, the Fe–TiO2, Fe2Ti2O7 and Ru–TiO2 were prepared according to the previous reports.4,5,8 The BiOBr catalyst with oxygen vacancies reported by Li was also prepared.21 The nitrogen photofixation abilities of these catalysts are shown in Fig. 10. Obviously, the activities of Fe–TiO2 and Fe2Ti2O7 are much lower than that of IM-CN(30). BiOBr also shows the lower N2 photofixation activity than that of IM-CN(30). The precious metal Ru doped TiO2 shows the comparable nitrogen photofixation ability to that of IM-CN(30). However, considering the high price of precious metal, IM-CN(30) is the best candidate among these catalysts.
 | ||
| Fig. 10 The comparison of nitrogen photofixation ability of Fe–TiO2, Fe2Ti2O7, Ru–TiO2, BiOBr and IM-CN(30) under visible light. | ||
Conclusions
A convenient infrared ray assisted microwave method for synthesizing graphitic carbon nitride (g-C3N4) with outstanding nitrogen photofixation ability under visible light is reported. Microwave treatment can form many irregular pores in the as-prepared g-C3N4, which causes the increased surface area and promoted separation rate of electrons and holes. Moreover, microwave treatment causes the formation of many nitrogen vacancies in the as-prepared g-C3N4. These nitrogen vacancies not only serve as active sites to adsorb and activate N2 molecules but also promote interfacial charge transfer from catalysts to N2 molecules, thus significantly improving the nitrogen photofixation ability. The higher nitrogen vacancies concentration of g-C3N4 prepared by infrared ray assisted microwave treatment causes the more chemical adsorption sites, leading to the higher nitrogen photofixation performance. More importantly, the present process is a convenient method for large-scale production of g-C3N4 which is significantly important for the practical application.Acknowledgements
This work was supported by Education Department of Liaoning Province (No. L2014145) and Environmental Science and Engineering Innovation Team of Liaoning Shihua University ([2014]-11).Notes and references
- M. E. Vol'pin, V. B. Shur and E. G. Berkovich, Inorg. Chim. Acta, 1998, 280, 264 CrossRef.
- G. J. Leigh, Science, 1998, 279, 506 CrossRef CAS.
- G. N. Schrauzer and T. D. Guth, J. Am. Chem. Soc., 1977, 99, 7189 CrossRef CAS.
- K. T. Ranjit, T. K. Varadarajan and B. Viswanathan, J. Photochem. Photobiol., A, 1996, 96, 181 CrossRef CAS.
- O. Rusina, O. Linnik, A. Eremenko and H. Kisch, Chem.–Eur. J., 2003, 9, 561 CrossRef CAS PubMed.
- K. Hoshino, Chem.–Eur. J., 2001, 7, 2727 CrossRef CAS.
- K. Hoshino, M. Inui, T. Kitamura and H. Kokado, Angew. Chem., Int. Ed., 2000, 39, 2509 CrossRef CAS.
- W. R. Zhao, J. Zhang, X. Zhu, M. Zhang, J. Tang, M. Tan and Y. Wang, Appl. Catal., B, 2014, 144, 468 CrossRef CAS.
- M. Kitano, Y. Inoue, Y. Yamazaki, F. Hayashi, S. Kanbara, S. Matsuishi, T. Yokoyama, S. W. Kim, M. Hara and H. Hosono, Nat. Chem., 2012, 4, 934 CrossRef CAS PubMed.
- A. Banerjee, B. D. Yuhas, E. A. Margulies, Y. B. Zhang, Y. Shim, M. R. Wasielewski and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 2030 CrossRef CAS PubMed.
- D. Zhu, L. H. Zhang, R. E. Ruther and R. J. Hamers, Nat. Mater., 2013, 12, 836 CrossRef CAS PubMed.
- D. V. Yandulov and R. R. Schrock, Science, 2003, 301, 76 CrossRef CAS PubMed.
- T. Xiong, W. L. Cen, Y. X. Zhang and F. Dong, ACS Catal., 2016, 6, 2462 CrossRef CAS.
- F. Dong, Z. W. Zhao, Y. J. Sun, Y. X. Zhang, S. Yan and Z. B. Wu, Environ. Sci. Technol., 2015, 49, 12432 CrossRef CAS PubMed.
- F. Dong, Y. H. Li, Z. Y. Wang and W. K. Ho, Appl. Surf. Sci., 2015, 358, 393 CrossRef CAS.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. Zhang, Z. Zhu, S. C. Smith, M. Jaroniec, G. Lu and S. Qiao, J. Am. Chem. Soc., 2011, 133, 20116 CrossRef CAS PubMed.
- J. Xu, H. T. Wu, X. Wang, B. Xue, Y. X. Li and Y. Cao, Phys. Chem. Chem. Phys., 2013, 15, 4510 RSC.
- Q. Li, J. Yang, D. Feng, Z. Wu, Q. Wu, S. S. Park, C. S. Ha and D. Zhao, Nano Res., 2010, 3, 632 CrossRef CAS.
- S. S. Park, S. W. Chu, C. Xue, D. Zhao and C. S. Ha, J. Mater. Chem., 2011, 21, 10801 RSC.
- Y. P. Yuan, L. S. Yin, S. W. Cao, L. N. Gu, G. S. Xu, P. W. Du, H. Chai, Y. S. Liao and C. Xue, Green Chem., 2014, 16, 4663 RSC.
- H. Li, J. Shang, Z. H. Ai and L. Z. Zhang, J. Am. Chem. Soc., 2015, 137, 6393 CrossRef CAS PubMed.
- S. Z. Hu, F. Y. Li, Z. P. Fan and J. Z. Gui, J. Power Sources, 2014, 250, 30 CrossRef CAS.
- G. G. Zhang, J. S. Zhang, M. W. Zhang and X. C. Wang, J. Mater. Chem., 2012, 22, 8083 RSC.
- M. Sun, T. Yan, Q. Yan, H. Y. Liu, L. G. Yan, Y. F. Zhang and B. Du, RSC Adv., 2014, 4, 19980 RSC.
- B. Yuan, Z. Y. Chu, G. Y. Li, Z. H. Jiang, T. J. Hu, Q. H. Wang and C. H. Wang, J. Mater. Chem. C, 2014, 2, 8212 RSC.
- M. Sun, Q. Yan, T. Yan, M. M. Li, D. Wei, Z. P. Wang, Q. Wei and B. Du, RSC Adv., 2014, 4, 31019 RSC.
- S. Z. Hu, L. Ma, H. Y. Wang, L. Zhang, Y. F. Zhao and G. Wu, RSC Adv., 2015, 5, 31947 RSC.
- M. Zhang, J. Xu, R. L. Zong and Y. F. Zhu, Appl. Catal., B, 2014, 147, 229 CrossRef CAS.
- B. Oregan and M. Gratzel, Nature, 1991, 353, 737 CrossRef CAS.
- Z. H. Hong, B. Shen, Y. L. Chen, B. Z. Lin and B. F. Gao, J. Mater. Chem. A, 2013, 1, 11754 CAS.
- R. C. Yang, X. J. Lu, X. Huang, Z. M. Chen, X. Zhang, M. D. Xu, Q. W. Song and L. T. Zhu, Appl. Catal., B, 2015, 170–171, 225 CrossRef CAS.
- Z. H. Wang, W. H. Ma, C. C. Chen, H. W. Ji and J. C. Zhao, Chem. Eng. J., 2011, 170, 353 CrossRef CAS.
- Y. W. Zhang, J. H. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300 RSC.
- L. Ge and C. Han, Appl. Catal., B, 2012, 117–118, 268 CrossRef CAS.
- W. Lei, D. Portehault, R. Dimova and M. Antoniettit, J. Am. Chem. Soc., 2011, 133, 7121 CrossRef CAS PubMed.
- X. Y. Li, D. S. Wang, G. X. Cheng, Q. Z. Luo, J. An and Y. H. Wang, Appl. Catal., B, 2008, 81, 267 CrossRef CAS.
- S. Z. Hu, F. Y. Li, Z. P. Fan, F. Wang, Y. F. Zhao and Z. B. Lv, Dalton Trans., 2015, 44, 1084 RSC.
- H. Q. Ma, S. Zhao, S. Li and N. Liu, RSC Adv., 2015, 5, 79585 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08817a |
| This journal is © The Royal Society of Chemistry 2016 |





