Composition-dependent structural and electrical properties of p-type SnOx thin films prepared by reactive DC magnetron sputtering: effects of oxygen pressure and heat treatment†
Abstract
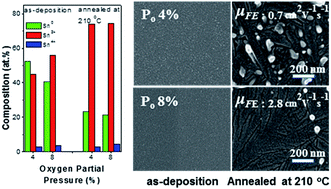
The composition-dependent structural and electrical properties of SnOx films prepared by means of reactive DC sputtering at various oxygen partial pressures (PO) and post-heat treatment temperatures (TA) were investigated, toward these films' potential use in p-channel oxide thin-film transistors (TFTs). A SnOx film fabricated under the lowest studied PO of 4% and heat-treated at 210 °C consisted of dendritic phases and irregular protrusions of metallic Sn. The resulting p-channel SnOx thin-film transistors suffered from marginal field effect mobility (μFE) and low on/off current ratio (ION/OFF), suggesting that the imperfect phases caused by oxygen-deficient stoichiometry hinder hole carrier conduction and act as bulk trap states. The heterogeneous structures observed in SnOx films annealed at 210 °C could be eliminated by increasing PO during fabrication. The resulting TFTs based on p-type SnOx films prepared at the high PO of 8% showed high mobilities up to 2.8 cm2 V−1 s−1 and reasonable ION/OFF of approximately 103, demonstrating the critical role of these films' homogeneous ordered aggregates without any imperfect phases such as a dendritic phase or irregular protrusions of metallic Sn. Among TFTs based on the films fabricated under 8% PO, the μFE and ION/OFF performance metrics degraded with increasing TA from 210 to 300 °C, which was mainly related to the 2SnO → SnO2 + Sn disproportionation reaction.

 Please wait while we load your content...
Please wait while we load your content...