Stereoselective synthesis of 4-aminobenzo[c][1,2]thiazine via modification of the Harmata benzothiazine synthesis†
S. R. K. Battulaab,
G. V. Subbareddyc,
I. E. Chakravarthyd and
V. Saravanan *a
*a
aMedicinal Chemistry, Jubilant Biosys Ltd, Yeshwanthpur, Bangalore, 560022, India. E-mail: v_saravanan@jubilantbiosys.com; sarav14@gmail.com
bDepartment of Chemistry, Jawaharlal Nehru Technological University, Ananthapur, 515002, Andhra Pradesh, India
cDepartment of Chemistry, Jawaharlal Nehru Technological University Ananthapur College of Engineering Pulivendula, 516390, YSR Dist, Kadapa, Andhra Pradesh, India
dDepartment of Chemistry, Rayalaseema University, Kurnool 518002, Andhra Pradesh, India
First published on 31st May 2016
Abstract
A stereoselective synthesis of 4-aminobenzothiazines was developed through a modified Harmata benzothiazine synthesis. The reaction has very good substrate scope and good functional group tolerance was observed. The products were obtained with high enantiomeric purity. Many interesting heterocyclic analogues were made using this methodology.
The utility of sulfoximines as chiral auxiliaries,1 chiral ligands2 and a C–H bond activating group has been well documented in organic synthesis literature.3 Many groups have published useful synthetic methodologies to produce various sulfoximine analogues.4 Two γ-glutamylcysteine synthetase inhibitors, methionine sulfoximine (MSO) and buthionine sulfoximine (BSO) have continued to attract the interest of the research community since their discovery in the year 1969.5 Despite this, the development of other compounds containing the sulfoximine moiety in medicinal chemistry has been rather slow. This is due to the fact that many syntheses of sulfoximines involved cumbersome routes, usage of hazardous chemicals and hampered by poor scalability. The interest resurged in recent times due to advances in safer methods to produce sulfoximines in large quantities with good enantiomeric purity.6 Attractive structural attributes of the sulfoximine moiety prompted many drug discovery teams to include it in their new compound designs. One such example is BAY 1000394, a pan-CDK inhibitor undergoing phase 1 clinical trials for treating patients with advanced solid tumors. This compound exhibited better ADME properties when compared to the corresponding sulfone derivative.7 Many more reports describe other biologically active sulfoximine compounds.8 In most of these compounds, the sulfoximine moiety appears as a linear structure rather than being part of a cyclic structure, except for few macrocycles.9 The less explored cyclic analogues could be very interesting to medicinal chemists due to their rigid three dimensional frame work and ease of further modification at various reaction centers.
There are several reports on the synthesis of bicyclic sulfoximines such as 1,2-benzothiazine and 2,1-benzothiazine. Harmata and co-workers have reported a synthesis of 1,2-benzothiazine by AlCl3-mediated cyclization of sulfonimidoyl chlorides with alkynes or alkenes and electrophilic cyclization of 2-bromophenyl substituted sulfoximines with terminal alkynes in the presence of palladium and copper catalysts.10 The same group has also reported one-pot synthesis of 2,1-benzothiazines through a palladium-catalyzed N-arylation of sulfoximines with o-bromo carbonyl compounds followed by intramolecular cyclization.11 This work was later extended to stereoselective intramolecular conjugate addition of sulfoximine carbanions to α,β-unsaturated functional groups.12 This in turn led to the synthesis of many useful natural products.13
Another class of 2-oxa-2-alkyl-3,4-dihydro-2,1-benzothiazine was synthesized by Bolm and co-workers via intramolecular palladium catalyzed N-arylation of appropriately functionalized sulfoximines with tethered 2-bromoaryl substituents.14 The same group has reported the synthesis of bicyclic 1,2-benzothiazine derivatives via a rhodium-catalyzed oxidative cyclization of phenyl sulfoximines with alkynes.3b,15 They have also subsequently reported a directed ortho alkenylation of phenyl sulfoximines with alkenes in the presence of a metal catalyst.16 Recently, Jeganmohan and co-workers reported the synthesis of tricyclic dibenzothiazines by a ruthenium-catalyzed ortho-arylation of phenyl sulfoximines with aromatic boronic acids followed by intramolecular cyclization in the presence of a palladium catalyst.17
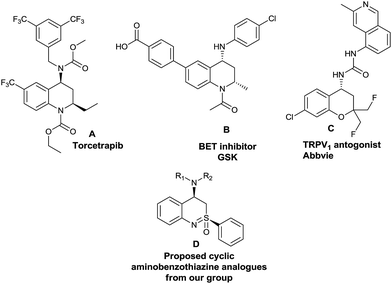
We were looking for new scaffolds containing the cyclic sulfoximine moiety to add to our compound library collection. We have become interested in the synthesis of 4-aminobezothiazines (compound D, Fig. 1), a compound class that has not been reported in the literature. This design was inspired by some topologically similar tetrahydroquinolin-4-amine and chroman-4-amine compounds that are biologically active (compounds A, B and C, Fig. 1).18
The proposed aminobenzothiazines would be a useful addition to existing cyclic sulfoximines. Here in, we present our results on the stereo selective synthesis of 4-aminobenzothiazines.
We envisaged that the synthesis of compound 6 could be achieved through 1,2-addition of a sulfoximine carbanion of compound 2 to N-(tert-butylsulfinyl)aldimine 1a followed by an intramolecular N-arylation reaction (Scheme 1).
N-(tert-Butylsulfinyl)aldimine (1a) was made from 2-bromobenzaldehyde and 2-methylpropane-2-sulfinamide. Reaction of compound 1a with N-TBDMS-protected sulfoximine 2 worked well with n-BuLi in THF at −78 °C. The crude product 3 was further subjected to deprotection of N-TBDMS with TBAF in THF at 0 °C to afford intermediate 4. An attempt was made to cyclize intermediate 4 to get N-(tert-butylsulfinyl)-4-aminobenzothiazine 5 under palladium and copper catalyzed conditions (Table 1). Unfortunately, the undesired 2,1-benzothiazine 7 was obtained as the major product through elimination of N-(tert-butylsulfinyl)amine group under palladium catalyzed conditions. The copper catalyzed conditions resulted in degradation of the starting material and gave mixture of unidentifiable products. The attempted reaction conditions were not found to be suitable for this intramolecular N-arylation reaction.
An alternate route that involves N-arylation as the first step and addition of sulfoximine carbanion as the second step was proposed (Scheme 2). This reaction, in principle should work similar to the methodology reported by Harmata and co-workers on the intra molecular conjugate addition of sulfoximine carbanions to α,β-unsaturated systems. To the best of our knowledge, there are no reports on intramolecular 1,2-addition of sulfoximine carbanions to sulfinimines. The stability of the phenylsulfinimines under the proposed reaction conditions would be a crucial factor in this reaction. N-Arylation of racemic sulfoximine 8 with the racemic sulfinimine compound 1a was attempted using Pd2(dba)3 and xanthphos in presence of Cs2CO3. To our satisfaction, the sulfinimine group was found to be stable under the attempted reaction conditions.
Compound 9 was then cyclized using various bases to obtain N-(tert-butylsulfinyl)-4-aminobenzothiazine 5. The best yield for the cyclization was obtained by generating the carbanion using LiHMDS as a base (Table 2). Upon deprotection of the tert-butylsulfinyl group compound 6 was obtained as a mixture of two enantiomers. The other stereo isomers were not formed under the present reaction conditions which implied that the intramolecular 1,2-addition is diastereoselective.
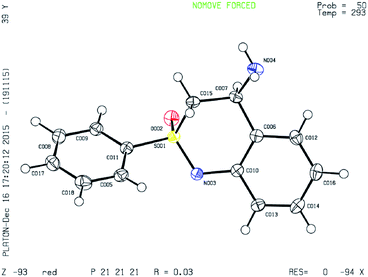
X-ray crystal structure of 6a (CCDC 1420064)
This was further confirmed by carrying out the cyclization of three different N-arylated sulfoximines (compound 9a, 9b and 9c, Scheme 3). These compounds had a fixed configuration at the sulfoximine moiety (S) but differed in the configuration of the tert-butylsulfinyl group. In compound 9a racemic tert-butylsulfinyl amine was used to make the sulfinimine. S and R tert-butylsulfinyl amines were used respectively to obtain corresponding sulfinimine compounds 9b and 9c. All the three reaction sequences led to the same final product namely (2S,4S)-4-amino-2-phenyl-3,4-dihydrobenzo[c][1,2]-thiazine-2-oxide 6a as a single isomer. The optical purity of compound 6a obtained from these three reactions was the same as revealed by data from chiral HPLC and optical rotation. This suggests that the stereochemical outcome of the reaction was mainly governed by the configuration of sulfoximine and has no dependency on chiral nature of sulfinimine.
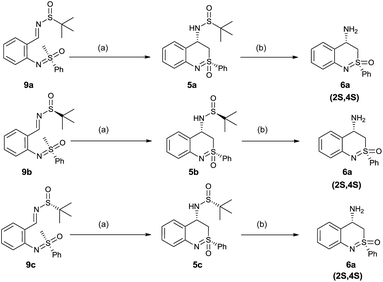 | ||
| Scheme 3 Stereoselective synthesis of 4-aminobenzothiazine. Reaction conditions: (a) 2.0 eq. LiHMDS (1 M) in THF, −78 °C, 2 h (b) (4 M) HCl/1,4-dioxane, MeOH, 0 °C, 2 h. | ||
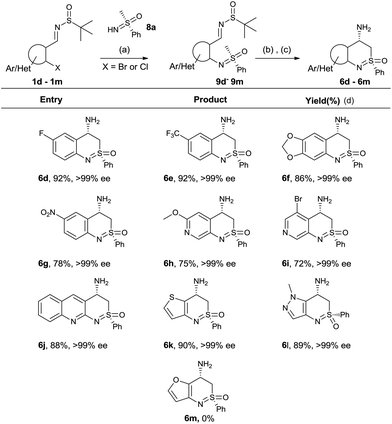
The absolute configuration of compound 6a was determined by single crystal X-ray diffraction. The substrate scope was further tested after establishing the reaction conditions. Various sulfinimines were prepared according to the known protocols for this purpose (compounds 1d–1m, Table 3). N-Arylation of sulfoximine 8a was performed using these sulfinimines (1d–1m). The corresponding N-arylated products were obtained in excellent yields (compounds 9d–9m, Table 3). Further cyclization and deprotection resulted in aminobenzothiazine compounds 6d–6l in excellent yields (Table 3). The absolute stereochemistry of the products was assigned analogous to the X-ray structure of compound 6a assuming that all the reactions follow the same pathway. The electronic nature of substituent on the aryl sulfinimines did not affect the product yields. This was evident from the yields obtained for compounds 6d to 6g (Table 3). The reaction worked well for heterocyclic substrates such as thiophene, pyridine, quinoline and pyrazole as well (compound 6h to 6l). Compounds 6g, 6i and 6h could be further derivatized due to presence of nitro, bromo and methoxy groups. Compound 6m which contains an acid labile furan group could not be synthesized under the present reaction conditions as the molecule disintegrated during the deprotection of tert-butyl sulfinyl group. More work is in progress to identify suitable reaction conditions for removal of the sulfinyl group or to change it to an easily cleavable protecting group.
| a Reaction conditions: (a) 5 mol% Pd2(dba)3, 7.5 mol% xanthphos, 2.0 equiv. Cs2CO3, 1,4-dioxane, 100 °C, 2 h (b) 2.0 eq. LiHMDS (1 M) in THF, −78 °C, 2 h (c) (4 M) HCl/1,4-dioxane, MeOH, 0 °C, 2 h. (d) Isolated yield. |
|---|
 |
In conclusion, a good reaction protocol was established to synthesize various chirally pure 4-aminobenzo[c][1,2]thiazine. Very good substrate scope and functional group tolerability were observed. Many interesting heterocyclic analogues were prepared. To further enhance the scope of the products, the resultant amines were converted to amide and urea analogues and are being tested against some biological targets. The results would be communicated in the future in a suitable forum.
References
- For selected examples, see: (a) C. R. Johnson, Acc. Chem. Res., 1973, 6, 341 CrossRef CAS; (b) M. Reggelin and C. Zur, Synthesis, 2000, 1 CrossRef CAS; (c) D. Craig, F. Grellepois and A. J. P. White, J. Org. Chem., 2005, 70, 6827 CrossRef CAS PubMed; (d) C. Moessner and C. Bolm, Org. Lett., 2005, 7, 2667 CrossRef CAS PubMed; (e) H. J. Gais, G. S. Babu, M. Günter and P. Das, Eur. J. Org. Chem., 2004, 7, 1464 CrossRef; (f) H. J. Gais, Heteroat. Chem., 2007, 18, 472 CrossRef CAS; (g) S. Koep, H. J. Gais and G. Raabe, J. Am. Chem. Soc., 2003, 125, 13243 CrossRef CAS PubMed; (h) M. Harmata and X. Hong, Org. Lett., 2007, 9, 2701 CrossRef CAS PubMed; (i) N. J. Peraino, K. A. Wheeler and N. Kerrigan, Org. Lett., 2015, 17, 1735 CrossRef CAS PubMed; (j) M. Nagatomo, S. Yoshioka and M. Inoue, Chem.–Asian J., 2015, 101, 120 CrossRef PubMed.
- For selected examples, see: (a) C. Bolm, M. Martin, O. Simic and M. Verrucci, Org. Lett., 2003, 5, 427 CrossRef CAS PubMed; (b) H. Okamura and C. Bolm, Chem. Lett., 2004, 33, 482 CrossRef CAS; (c) C. Bolm, M. Verrucci, O. Simic, P. G. Cozzi, G. Raabe and H. Okamura, Chem. Commun., 2003, 2826 RSC; (d) C. Bolm and O. Simić, J. Am. Chem. Soc., 2001, 123, 3830 CrossRef CAS PubMed; (e) M. Langner and C. Bolm, Angew. Chem., Int. Ed., 2004, 43, 5984 CrossRef CAS PubMed; (f) M. Langner, P. Rémy and C. Bolm, Chem.–Eur. J., 2005, 11, 6254 CrossRef CAS PubMed; (g) C. Moessner and C. Bolm, Angew. Chem., Int. Ed., 2005, 44, 7564 CrossRef CAS PubMed; (h) M. Frings, I. Thomé, I. Schiffers, F. Pan and C. Bolm, Chem.–Eur. J., 2014, 20, 1691 CrossRef CAS PubMed.
- For selected examples, see: (a) R. K. Rit, M. R. Yadav and A. K. Sahoo, Org. Lett., 2012, 14, 3724 CrossRef CAS PubMed; (b) W. Dong, L. Wang, K. Parthasarathy, F. Pan and C. Bolm, Angew. Chem., Int. Ed., 2013, 52, 11573 CrossRef CAS PubMed; (c) R. M. Yadav, R. K. Rit, M. Shankar and A. K. Sahoo, J. Org. Chem., 2014, 79, 6123 CrossRef PubMed; (d) Y. Cheng and C. Bolm, Angew. Chem., Int. Ed., 2015, 54, 12349 CrossRef CAS PubMed; (e) M. R. Yadav, M. Shankar, E. Ramesh, K. Ghosh and A. K. Sahoo, Org. Lett., 2015, 17, 1886 CrossRef CAS PubMed.
- For selected examples, see: (a) F. Teng, J.-T. Yu, Z. Zhou, H. Chu and J. Cheng, J. Org. Chem., 2015, 80, 2822 CrossRef CAS PubMed; (b) F. Teng, J. Cheng and J.-T. Yu, Org. Biomol. Chem., 2015, 13, 9934 RSC; (c) F. Teng, S. Sun, Y. Jiang, J.-T. Yu and J. Cheng, Chem. Commun., 2015, 51, 5902 RSC; (d) R. Pirwerdjan, P. Becker and C. Bolm, Org. Lett., 2015, 17, 5008 CrossRef CAS PubMed; (e) S. Kim, J. Kim, J. Lee and P. H. Lee, Adv. Synth. Catal., 2015, 357, 3773 CrossRef CAS; (f) C. Bohnen and C. Bolm, Org. Lett., 2015, 17, 3011 CrossRef CAS PubMed; (g) Z. Zhao, T. Wang, L. Yuan, X. Jia and J. Zhao, RSC Adv., 2015, 5, 75386 RSC; (h) F. Teng, J. Cheng and C. Bolm, Org. Lett., 2015, 17, 3166 CrossRef CAS PubMed; (i) F. Teng, J.-T. Yu, Y. Jiang, H. Yang and J. Cheng, Chem. Commun., 2014, 50, 8412 RSC.
- (a) O. W. Griffith, M. E. Anderson and A. Meister, J. Biol. Chem., 1979, 254, 1205 CAS; (b) J. M. Manning, S. Moore, W. B. Rowe and A. Meister, Biochemistry, 1969, 8, 2681 CrossRef CAS PubMed.
- For selected examples, see: (a) V. Bizet, C. M. M. Hendriks and C. Bolm, Chem. Soc. Rev., 2015, 44, 3378 RSC; (b) B. Gutmann, P. Elsner, A. O'Kearney-McMullan, W. Goundry, D. M. Roberge and O. C. Kappe, Org. Process Res. Dev., 2015, 19, 1062 CrossRef CAS; (c) M. Zenzola, R. Doran, R. Luisi and J. A. Bull, J. Org. Chem., 2015, 80, 6391 CrossRef CAS PubMed; (d) S. Dong, M. Frings, H. Cheng, J. Wen, D. Zhang, G. Raabe and C. Bolm, J. Am. Chem. Soc., 2016, 138, 2166 CrossRef CAS PubMed; (e) H. Lebel, H. Piras and M. Borduy, ACS Catal., 2016, 6, 1109 CrossRef CAS.
- (a) U. Lücking, G. Siemeister, P. Lienau, R. Jautelat and J. SchulzeBayer Schering Pharma Aktiengesellschaft, EP 2179991, 2010; (b) U. Lücking, R. Jautelat, M. Krüger, T. Brumby, P. Lienau, M. Schüfer, H. Briem, J. Schulze, A. Hillisch, A. Reichel and G. Siemeister, ChemMedChem., 2013, 8, 1021 CrossRef.
- (a) U. Lücking, Angew. Chem., Int. Ed., 2013, 52, 9399 CrossRef PubMed; (b) U. Lücking, N. Böhnke, A. Scholz, P. Lienau, G. Siemeister, U. Bömer, D. Kosemund and R. Bohlmann, Bayer Schering Pharma Aktiengesellschaft, WO 2014076091, 2014; (c) M. Blum, D. Gottschling, A. Heckel, J. Hehn, T. Lehmann, and D. Widenmayer, Boehringer Ingelhein International GMBH, WO 2014072244, 2014; (d) S. Boral, S. Wang and J. Wurster, Aller-Gan, INC, WO 2014072244, 2014.
- (a) S. Bailey, B. J. Burke, M. R. Collins, J. J. Cui and J. G. Deal, Pfizer Inc, WO2013132376, 2013; (b) S. Yuntao and J. B. Alexander, Cs Therapeutics Inc, WO2015050989, 2015.
- (a) M. Harmata and E. O. Schlemper, Tetrahedron Lett., 1987, 28, 5997 CrossRef CAS; (b) M. Harmata, R. J. Claassen and C. L. Barnes, J. Org. Chem., 1991, 56, 5059 CrossRef CAS; (c) M. Harmata and M. Kahraman, J. Org. Chem., 1998, 63, 6845 CrossRef CAS PubMed; (d) M. Harmata, M. Kahraman, D. E. Jones, N. Pavri and S. E. Weatherwax, Tetrahedron, 1998, 54, 9995 CrossRef CAS; (e) M. Harmata, K. Rayanil, M. G. Gomes, P. Zheng, N. L. Calkins, S.-Y. Kim, Y. Fan, V. Bumbu, D. R. Lee, S. Wacharasindhu and X. Hong, Org. Lett., 2005, 7, 143 CrossRef CAS PubMed.
- (a) M. Harmata and N. Pavri, Angew. Chem., Int. Ed., 1999, 38, 2419 CrossRef CAS; (b) C. Bolm and J. P. Hildebrand, Tetrahedron Lett., 1998, 37, 5731 CrossRef; (c) M. Harmata and S. K. Ghosh, Org. Lett., 2001, 3, 3321 CrossRef CAS PubMed.
- (a) M. Harmata and X. Hong, J. Am. Chem. Soc., 2003, 125, 5754 CrossRef CAS PubMed; (b) M. Harmata, K. Rayanil, V. R. Espejo and C. L. Barnes, J. Org. Chem., 2009, 74, 3214 CrossRef CAS PubMed.
- (a) M. Harmata, X. Hong and C. L. Barnes, Tetrahedron Lett., 2003, 44, 7261 CrossRef CAS; (b) M. Harmata, X. Hong and P. R. Schreiner, J. Org. Chem., 2008, 73, 1290 CrossRef CAS PubMed; (c) M. Harmata and X. Hong, Tetrahedron Lett., 2005, 46, 3847 CrossRef CAS; (d) M. Harmata, X. Hong and C. L. Barnes, Org. Lett., 2004, 6, 2201 CrossRef CAS PubMed; (e) M. Harmata and X. Hong, Org. Lett., 2005, 7, 3581 CrossRef CAS PubMed; (f) M. Harmata, Z. Cai and Y. Chen, J. Org. Chem., 2009, 74, 5559 CrossRef CAS PubMed.
- A. G. Pandey, M. J. McGrath, O. G. Mancheño and C. Bolm, Synthesis, 2011, 23, 3827 Search PubMed.
- Y. Cheng and C. Bolm, Angew. Chem., Int. Ed., 2015, 51, 12349 CrossRef PubMed.
- (a) K. Parthasarathy and C. Bolm, Chem.–Eur. J., 2014, 20, 4896 CrossRef CAS PubMed; (b) W. Dong, K. Parthasarathy, Y. Cheng, F. Pan and C. Bolm, Chem.–Eur. J., 2014, 20, 15732 CrossRef CAS PubMed.
- R. K. Chinnagolla, A. Vijeta and M. Jeganmohan, Chem. Commun., 2015, 51, 12992 RSC.
- (a) D. B. Damon, R. W. Dugger and R. W. Scott, U.S. pat., 6, 689, 897, 2004; (b) D. B. Damon, R. W. Dugger, S. E. Hubbs, J. M. Scott and R. W. Scott, Org. Process Res. Dev., 2006, 10, 472 CrossRef CAS; (c) R. Gosmini, V. Nguyen, J. Toum, C. Simon, J. G. Brusq, G. Krysa, O. Mirguet, A. M. Riou-Eymard, E. V. Boursier and L. Trottet, J. Med. Chem., 2014, 57, 8111 CrossRef CAS PubMed; (d) E. A. Voight, A. R. Gomtsyan, J. F. Daanen, R. J. Perner, R. G. Schmidt, E. K. Bayburt, S. DiDomenico, H. A. McDonald, P. S. Puttfarcken and J. Chen, J. Med. Chem., 2014, 57, 7412 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1420064. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra08590c |
| This journal is © The Royal Society of Chemistry 2016 |