Radical exchange reaction of multi-spin isoindoline nitroxides followed by EPR spectroscopy†
Abstract
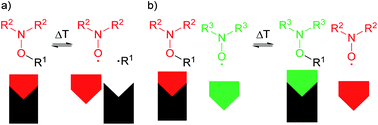
The synthesis of a rigid, isoindoline-functionalized tetraphenylmethane multi-spin system is described. The isoindoline nitroxide groups are used in a nitroxide exchange reaction with a TEMPO containing alkoxyamine. Using EPR spectroscopy it is possible to follow the exchange process and thereby find the optimal experimental conditions to have the maximum yield. The presented approach could be used to study the nitroxide exchange process of various systems and to determine the kinetics of the exchange process. The presented molecular components can be used as tectons in the construction of covalently linked organic networks or as model systems for EPR distance measurements.

 Please wait while we load your content...
Please wait while we load your content...