Osteochondral scaffold combined with aligned nanofibrous scaffolds for cartilage regeneration
Paul Leea,
Ohan S. Manoukianbd,
Gan Zhoua,
Yuhao Wanga,
Wei Changa,
Xiaojun Yua and
Sangamesh G. Kumbar*bcd
aDepartment of Biomedical Engineering, Chemistry and Biological Sciences, Stevens Institute of Technology, 1 Castle Point on Hudson, Hoboken, NJ 07030, USA
bDepartment of Biomedical Engineering, University of Connecticut, 97 North Eagleville Road, Storrs, CT 06269, USA
cDepartment of Materials Science and Engineering, University of Connecticut, 97 North Eagleville Road, Storrs, CT 06269, USA
dDepartment of Orthopaedic Surgery, University of Connecticut Health Center, 263 Farmington Ave, Farmington, CT 06030, USA. E-mail: kumbar@uchc.edu
First published on 21st July 2016
Abstract
Osteochondral defect repair poses a significant challenge in its reconstruction as the damage is presented in both articular cartilage and the underlying subchondral bone. Tissue engineering approaches have utilized various scaffolds in combination of stem cells and growth factors to regenerate the defect. Still a significant challenge remains in creating a scaffold structure that supports the proliferation and differentiation of bone marrow stromal cells (BMSCs) into chondrocytes and osteoblasts while providing the appropriate mechanical stability. The present manuscript reports the fabrication and characterization of a biphasic scaffold system derived from biodegradable polymers such as poly(lactic acid-glycolic acid) (PLGA) as a hard shell and polycaprolactone (PCL) a soft component. Collectively this biphasic scaffold was able to withstand physiological loads up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in a cyclic compressive test. The scaffold surface was decorated with PCL aligned nanofibers contacting chondroitin sulfate and hyaluronic acid and nanofibers were cross-linked via carbodiimide linkages to retain these bioactive molecules over the culture period. The present study aims to show the potential of these bioactive scaffolds for the repair of osteochondral defects. Scaffolds were characterized by Fourier transform infra-red spectroscopy, optical microscopy and cyclic compressive testing. Primary rat bone marrow stem cells were seeded onto scaffolds and cell proliferation and differentiation was evaluated using RTPCR and immunohistochemistry. RT-PCR indicated that the scaffold was able to stimulate the different regions of osteochondral tissue: collagen type II and aggrecan expression in the cartilage region and BMP-2 in the bone region. Similarly protein secretion with induced alignment was confirmed with immunofluorescence imaging. This novel hybrid scaffold shows promising results in the regeneration of cartilage tissue as well as the underlying subchondral bone.
000 cycles in a cyclic compressive test. The scaffold surface was decorated with PCL aligned nanofibers contacting chondroitin sulfate and hyaluronic acid and nanofibers were cross-linked via carbodiimide linkages to retain these bioactive molecules over the culture period. The present study aims to show the potential of these bioactive scaffolds for the repair of osteochondral defects. Scaffolds were characterized by Fourier transform infra-red spectroscopy, optical microscopy and cyclic compressive testing. Primary rat bone marrow stem cells were seeded onto scaffolds and cell proliferation and differentiation was evaluated using RTPCR and immunohistochemistry. RT-PCR indicated that the scaffold was able to stimulate the different regions of osteochondral tissue: collagen type II and aggrecan expression in the cartilage region and BMP-2 in the bone region. Similarly protein secretion with induced alignment was confirmed with immunofluorescence imaging. This novel hybrid scaffold shows promising results in the regeneration of cartilage tissue as well as the underlying subchondral bone.
Introduction
Osteoarthritis (OA) is a devastating disease affecting almost one tenth of the American population. It is amongst the leading causes of disability inflicting an annual cost of $65 billion on the medical system.1–3 Though there are many methods to regenerate cartilage, none so far have been successful enough to be considered the “gold standard”. Current treatments often involve autografts, which are plagued with supply issues and require additional surgery, or allografts, which can have issues with supply and difficulty in processing. Synthetic materials, though abundantly available, are not as bioactive and still rely on cells to not only populate the scaffold, but also deposit the extracellular matrix (ECM). For cartilage regeneration, autologous osteochondral transfer has been the standard treatment, however, it is still not optimal due to its need to create another defect within the patient. Another popular technique is microfracture, which involves fracturing the subchondral surface to release bone marrow stem cells (BMSCs).4 Several issues are associated with this treatment including production of inferior fibrocartilage, which is characterized by an abundance of collagen type I and a lack of collagen type II, due to the lack of a three dimensional scaffold, environmental cues, and mechanical protection. Typical hyaline cartilage found in the articular region is characterized by collagen type II.5–9Unlike cartilage-only scaffolds, which are prone to delamination, an osteochondral scaffold can overcome the separation of the phases by simultaneously healing bone and cartilage.10–12 Because a cartilage only scaffold does not drill down into the boney layer it cannot initiate bleeding of the bone marrow, thus there will be no cells to seed the scaffold. Autologous chondrocyte implantation (ACI), one of the latest is another benchmark treatment, which involves culturing chondrocytes ex vivo and then re-implanting them back into the defect site. With ACI there is still risk of delamination and it still does not provide an adequate scaffold.13,14 Though there are several scaffolds that use the ACI process, they still require multiple procedures, one to harvest chondrocytes, and another to seed and implant. By combining an osteochondral scaffold with the aforementioned microfracture technique, these issues can be overcome to create a one step process.
One of the most unique features about articular cartilage is the three dimensional (3D) layout of the ECM. It is comprised of three different layers: a superficial layer characterized by higher concentration of collagen with fibrils arranged parallel to the surface movement to provide resistance to shear stress; a medial zone, where the cells and fibrils are randomly oriented to transition the stresses to the lower layer; and the radial zone where the cells and collagen fibrils are arranged perpendicular to the joint movement provide compressive stress resistance.5,15–17 This anisotropy is what gives cartilage its enhanced mechanical properties to assist in pain-free movement of the joints.
Previous experiments using electrospun polymeric nanofibers to recreate the unique articular cartilage ECM were replicated to be combined with an outer shell and a secondary alignment of fibers. With a spiral porous polymer sheet, the surface area can be increased for greater amounts of nanofiber deposition, while scaffold volume can be simultaneously decreased.18,19 The spiral shape permits the electrospinning of the nanofibers on the entire surface of a polymer sheet, then by rolling the sheet into a spiral, it forms a 3D shape, resulting in a scaffold that is not only composed primarily of nanofibers, but encourages cellular infiltration.
Though polycaprolactone (PCL) is a commonly used polymer, it suffers from reduced bioactivity, due to issues such as poor wettability, which affects cellular attachment.20 In order to increase cellular attachment and aid BMSC differentiation, chondroitin sulfate (CS) and hyaluronic acid (HYA), both natural components of cartilage, have been added. From our previous experiments, collagen type II and aggrecan secretion from BMSCs was found to be regulated through the addition of CS, HYA, and nanofiber alignment.21,22 As seen from previous experiments, hydroxyapatite (HA) has been shown to initiate differentiation of BMSCs into osteoblast while increasing collagen type I, osteopontin (OPN), osteocalcin (OCN), osteonectin (ON), bone morphogenetic protein-2 (BMP-2), and alkaline phosphatase (ALP) secretion, all proteins associated with bone regeneration.23–27
By combining the nanofibrous scaffolds with a sintered microsphere shell, the growing cartilage can be protected from environmental stresses while anchoring the scaffold in place. The porous microsphere structure allows for cellular infiltration and can help resist repetitive compressive stresses that can eventually wear down a scaffold.28,29 The use of composite PCL with poly(lactic-co-glycolic acid) (PLGA) combines the stronger mechanical properties of PLGA with the longer degradation period of PCL. To increase attachment and chondrogenesis on the shell, CS and HYA were both grafted onto the cartilage region of the outer shell.
The osteochondral autologous transfer system (OATS) usually consists of osteochondral plugs of around 8 mm in diameter which was found to be the most stable sized plug.30 To match typical osteochondral plugs, 3 mm of the scaffold will be designated for the cartilage regeneration layer, while 9 mm of the scaffold will be for bone regeneration to match that of the subchondral bone.31,32 The novel scaffold was designed with a central channel to allow for BMSC migration from the lower subchondral area into the cartilage regeneration area and to provide access for the flow of blood and nutrients (Fig. 1).
Methods and materials
Porous sheet scaffold fabrication
PCL and NaCl particles both from Sigma-Aldrich (Sigma-Aldrich Co., St. Louis, MO), where the NaCl was ground and sieved to average in size from 100 to 150 μm to be used in porosity creating via salt leeching. In short, NaCl was laid across a glass mold to create a bottom porous structure, then PCL dissolved in dichloromethylene (DCM) (Pharmco-AAPER, Brookfield, CT) at an 8% (w/v) solution and poured into the glass mold accordingly to our previously published methods.18,19,33 The same NaCl particles were spread across the top of the casted polymer solution to form pore interconnection. Once all the DCM evaporated, the solution casted sheets were salt leached in diH2O overnight and then dried. The dried PCL sheet was cut into 3 by 50 mm sheets for the cartilage area. For the bone forming spiral sheets, 20% (w/w) HA (Sigma-Aldrich Co., St. Louis, MO) was blended into the PCL solution before casting. For the bone spiral scaffold sheets were cut into 9 mm by 40 mm strips.Nanofiber fabrication
The PCL nanofiber solution was created by combining an 8% (w/v) solution of PCL in hexaflouro-2-propanol (HFIP, Oakwood Products, Inc., Estill, SC) with a 10% (v/v) aqueous solution containing 20% CS and 0.2% HYA (w/w). The aqueous solution was used to dissolve the CS and HYA before blending to PCL in the same fashion as our previous study.21 For the bone forming area, instead of HYA and CS, 20% (w/w) HA was blended into the 8% PCL solution. Similar to from the previous studies, electrospinning was performed with a 20G needle, working distance of 10 cm, 12 kV potential voltage, and a flow rate of 0.4 ml h−1.21,22,33To create the aligned nanofibers, the PCL strips will be situated on top of a glass slide which edges are placed on two parallel metal plates, thus allowing it to float in the air. The metal plates direct the fibers to vertically align on the PCL sheets.21,34,35 Using the same protocol as before, fibers were electrospun for 6 minutes for each side. Afterwards, the sheets were rolled into a spiral shape and then held in shape with copper shims at 40 °C for 1 hour for shape retention. For the plain osteochondral scaffold, only PCL without HA, CS and HYA was used.
Microsphere fabrication
Similar to one of our previous studies, 75% PLGA 25% PCL (w/w) composite microsphere were made using an oil in water emulsion technique.36 In short the PLGA PCL pellets were dissolved in DCM in a 10% (w/v) solution and then injected using a 20 G needle into a stirring 1% (w/v) poly vinyl alcohol (PVA, Sigma, MO) solution and left overnight to ensure DCM evaporation. The microspheres were then placed into a filter paper with suction and washed with diH2O to ensure no excess PVA remained. Once dried, the microspheres were filtered by size with a sieve from 250 to 350 μm.A Teflon mold with a diameter of 8 mm and height of 12 mm was used for scaffold fabrication. The inner diameter was 5 mm for the cartilage regeneration area and 4 mm for the bone regeneration are. This allowed for 1.5 mm walls in the cartilage formation area and 2 mm walls for the bone forming area. The mold was filled with the microspheres and sintered at 75 °C for 4 hours. After cooling, the spiral bone and cartilage scaffolds were inserted into each side of the outer shell.
Horizontally aligned nanofibers
Horizontally aligned PCL nanofibers with 10% (w/v) CS and (w/v) 0.5% HYA were electrospun onto the top of cartilage portion of the scaffold for 6 minutes at 10 kV with a flow rate of 0.4 ml h−1. The nanofibers were then cut so that the fibers could be folded over the side covering 3 mm of the outside. Similar to the first set of nanofibers, the plain osteochondral scaffold had PCL fibers spun on top.Chondroitin sulfate and hyaluronic acid grafting and crosslinking
To increase attachment and chondrogenesis on the outer shell in the upper cartilage area, CS and HYA were grafted by immersing the cartilage region in a 20% (w/v) CS and 1% HYA (w/v) solution for 1 hour. The scaffold was then removed from the solution and left to dry overnight. Once dried, the area with CS and HYA were crosslinked using a technique described in our previous work.21 In short, the scaffolds were crosslinked via 48 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (Sigma, St. Louis, MO) and 6 mM N-hydroxysuccinimide (NHS) (Sigma, St. Louis, MO) (w/v) 50 mM 2-(N-morpholino) ethanesulfonic acid (MES) (Sigma, St. Louis, MO) buffer for 24 hours at 37 °C. Afterwards, the scaffolds were triple washed with DI water. The plain osteochondral scaffold was only subjected to the crosslinking process and not the immersion into the CS and HYA solution.FTIR characterization
In addition to the previous glycosaminoglycan assay to confirm the presence of CS and HYA after crosslinking and immersion in PBS, Fourier transform infrared spectroscopy (FTIR) with a Tensor 27 (Bruker, Billerica, MA) was performed at all steps involving crosslinking by separating the cartilage and bone sections of the scaffold and dissolving each in DCM separately.21,22,33 Each sample was measured to be 1 mg and combined with 250 mg of potassium bromide (Sigma, St. Louis, MO) and pressed to form the pellet. Once completely dried the mixture was made into the pellet and ran with a minimum of 32 scans with an average scan resolution of 32 cm−1 within the 400 to 4000 cm−1 range.Mechanical testing
Two different types of compressive mechanical testing were carried out to determine whether the scaffold was mechanically feasible. Ultimate yield compressive stress testing was determined by crushing the complete osteochondral scaffold in wet confined conditions with a 100 N load cell and a strain rate of 0.1 mm min−1 (Instron, Norwood, MA). Durability testing was carried out with cyclic testing in a Bose Enduratec with a 9.8 MPa load at 1 Hz in confined conditions with PBS wetting for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, similar to the upper limit of physiological loading on articular cartilage.37
000 cycles, similar to the upper limit of physiological loading on articular cartilage.37
BMSC culture, seeding, and proliferation
Rat BMSCs (Lonza, Basel, CH) were cultured using BSMC medium also from Lonza until the third passage in a T-175 flask (USA Scientific, Orlando, FL), according to previous methods as done by Li, et al.38 After sterilization of the osteochondral scaffolds with 70% (v/v) isopropyl alcohol and a triple rinse with DI water, the scaffolds were immersed in Dulbecco's Modified Eagle's Medium (DMEM) (Gibco, Grand Island, NY) with 10% FBS (v/v), 1% Penicillin/streptomycin (v/v) (ATCC, Manassas, VA).Trypsinized BMSCs were seeded onto both a plain osteochondral scaffold without CS, HYA, HA and a complete scaffold with CS, HYA, and HA at a density of 5 × 105 cells per scaffold. The same medium used to incubate the scaffold was used and changed every other day. Attachment, proliferation, and cell viability was measured using (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) assay (Promega, Madison, WI) at days 1, 7, 14, and 28 with an N = 5. The assay was performed on the tissue culture plate (TCP), plain osteochondral, and complete osteochondral scaffold. All cell cultures were cultured in a humidified incubator at 37 °C with 5% CO2.
Gene expression
Expression of the chondrogenic and osteogenic genes was determined via reverse transcriptase polymerase chainreaction (RT-PCR), where at day 28, RNA was extracted from each sample using the Qiagen RNA MiniPrep Kit (Qiagen, Netherlands) (N = 5). Each scaffold was removed from culture then split at the bone and cartilage area junction 3 mm from the cartilage side. Each part was then mixed with the lysis buffer within a 10 ml syringe with an 18 G needle, where the scaffold was crushed and mixed with the lysis solution by pushing the plunger up and down several times to ensure complete RNA extraction.Concentration was determined using the ND-1000 spectrophotometer (N = 5) (Nanodrop, Wilmington, DE). 2 μg of RNA was transcribed into cDNA using M-MLV Reverse Transcriptase (Promega, Madison, WI) and OligoDT (Invitrogen, Carlsbad, CA) accordingly to manufacturers' instructions. The resulting cDNA was combined with Green Mix (Promega, Madison, WI) with the specific primers as listed in Table 1. Final DNA products were resolved via electrophoresis with an 8% agarose gel with imaging performed with a UVP Photo-Doc Imaging System (UVP, Upland, CA), where all gels were run with a 1 Kb Plus DNA Ladder (Invitrogen, Grand Island, NY).
| Forward | Reverse | Amplicon length | Accession # | |
|---|---|---|---|---|
| GAPDH | GTCTACTGGCGTCTTCACC | AGGGATGATGTTCTGGGCTG | 334 | NM_017008.4 |
| Aggrecan | AACTTCTTCGGAGTGGGTGG | CTGCTGTGCCTCCTCAAATG | 444 | NM_022190.1 |
| BGLAP | CAAGTCCCACACAGCAACTC | GTCCATTGTTGAGGTAGCGC | 204 | NM_013414.1 |
| BMP-2 | GAAGCCAGGTGTCTCCAAGAG | GTGGATGTCCTTTACCGTCGT | 142 | NM_017178.1 |
| BSP | GATAGTTCGGAGGAGGAGGG | CTAACTCCAACTTTCCAGCGT | 172 | NM_012587 |
| Collagen type I | TGTTCGTGGTTCTCAGGGTAG | TTGTCGTAGCAGGGTTCTTTC | 280 | NM_053356.1 |
| Collagen type II | CTGCTCCTAGAGCCTCCTGC | GCCCTAATTTTCGGGCATCC | 211 | NM_012929.1 |
| Collagen type IV | AGGACCAAAAGGCGACAAG | ACCGGGCTCTCCTCTTAACC | 403 | NM_001135009.1 |
| COMP | GACAAGATCGATGTGTGCCC | ACCGTTGAATGCCGTGTAAC | 195 | NM_012834.1 |
| OPN | GGAGTCCGATGAGGCTATCAA | TCCGACTGCTCAGTGCTCTC | 189 | M99252.1 |
| Runx2 | ACGTACCCAGGCGTATTTCA | GCTGGATAGTGCATTCGTGG | 187 | NM_001278483.1 |
| Sox-9 | GGGCTCGCGTATGAATCTCC | GCTTGACGTGTGGCTTGTTC | 329 | XM_001081628.3 |
Immunofluorescence imaging
To determine morphological anisotropy of the different proteins secreted, immunofluorescence imaging was performed with confocal microscopy (Zeiss LSM 5, CH) at day 28. The spiral scaffolds were removed from the osteochondral shell and rolled out flat. Care was taken to not disorient the top nanofibers. All samples were triple rinsed with PBS, then immersed in warm 4% paraformaldehyde overnight at 4 °C. The paraformaldehyde was then rinsed again with PBS and then the scaffolds were immersed in a blocking and permeabilization solution of 3% bovine serum albumin (BSA, MP Biomedicals, Santa Ana, CA) in a tris-buffered saline with 1% Tween (TBST, Sigma, St. Louis, MO) solution for 1.5 hours at room temperature.Following blocking and permeabilization, scaffolds were subsequently incubated in rabbit polyclonal 1/250 anti-aggrecan antibody (ab36861), 1/250 anti-collagen type I (ab34710), 1/250 anti-collagen type II (ab34712), 1/250 anti-osteopontin (ab8448), and 1/250 anti-BMP-2 (ab14933) all from Abcam (Abcam, Cambridge, United Kingdom) in TBST with 3% BSA overnight at 4 °C under slight agitation. Afterwards, they were rinsed 3 times with TBST, then incubated with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Alexafluor 488 Goat anti-rabbit IgG (Life Technologies, Norwalk, CT) in 3% BSA in TBST at room temperature in the dark for 1.5 hours, then triple rinsed. Nucleus and mounting was done with Fluoroshield with DAPI (Sigma, St. Louis, MO). After curing for 20 minutes in the dark, images were taken using confocal microscopy at 20×.
500 Alexafluor 488 Goat anti-rabbit IgG (Life Technologies, Norwalk, CT) in 3% BSA in TBST at room temperature in the dark for 1.5 hours, then triple rinsed. Nucleus and mounting was done with Fluoroshield with DAPI (Sigma, St. Louis, MO). After curing for 20 minutes in the dark, images were taken using confocal microscopy at 20×.
Statistical analysis
Statistical analysis of MTS was performed with students T-test p values less than 0.05 considered significantly different. All data was reported as a mean ± standard deviation.Results
Scaffold fabrication
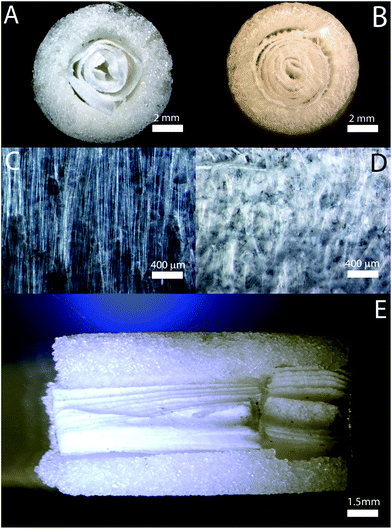
In previous publications, the inner spiral portions of the scaffold was subjected to in vitro testing and it was shown that the alignment of nanofibers can be preserved throughout the entire culture period and the manipulation in rolling it into a spiral form. Fig. 2 shows the different views of the scaffold, where Fig. 2C and D are the optical micrographs of the differently aligned nanofibers, aligned, and random, respectively. Fig. 2C shows that the nanofibers remain intact and keep their orientation even after crosslinking. In both cartilage and bone sides, the spiral structure of the scaffold can be seen. Note: a disconnect between the two scaffold components inner cartilage and bone spiral structures exists due to the process of making the scaffold.FTIR characterization
Confirmation of different stages of fabrication and crosslinking of the scaffold after washing and sterilization was performed with FTIR. Fig. 3A–D show the control spectra of PCL, CS, HYA, and HYA. Peaks such as 1170 and 1730 cm−1 indicate the C–O and C=O bonds of PCL. For chondroitin sulfate (Fig. 3B), the spectra shows peaks at 3400, 1246 and 1040 cm−1 of the amine, sulfate and ether functional groups. 3400, 1650, 1570 and 1040 cm−1 show the peaks for the amine, amide, carboxylate and ether groups of HYA (Fig. 3C). The phosphate groups are represented by the peaks at 1100 and 1040 cm−1 in the HA control (Fig. 3D). To show conservation of the CS and HYA after crosslinking the FTIR spectra (Fig. 3E) shows same characteristic peaks of CS and HYA at 3400, 1570 and 1246 cm−1 peaks of the amine, carboxylate and sulfate groups. The sample of PCL with HA shows that after sterilization, the phosphate peaks at 1100 and 1040 cm−1 appeared in the spectra (Fig. 3F).Cyclic compressive testing
Due to the nature of cartilage being a load-bearing tissue with repetitive stresses, it was important to determine the capability of the scaffold to resist such mechanical and physiological stresses. The scaffold was subjected to a 9.8 MPa load for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles or a 50 N load on a 50.25 mm2 area. The physiological load showed that the scaffold remained intact at 10
000 cycles or a 50 N load on a 50.25 mm2 area. The physiological load showed that the scaffold remained intact at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and that it was able to reach its resting status at around 3000 cycles at which strain was very low. From 0 to 3000 cycles there was about 0.06 mm of compression from 0.1 to 0.04 mm resulting in a strain of 0.5% (Fig. 4).
000 cycles and that it was able to reach its resting status at around 3000 cycles at which strain was very low. From 0 to 3000 cycles there was about 0.06 mm of compression from 0.1 to 0.04 mm resulting in a strain of 0.5% (Fig. 4).
 | ||
Fig. 4 Cyclic loading of the complete osteochondral scaffold at 9.8 MPa for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles exhibiting asymptotic behaviour. 000 cycles exhibiting asymptotic behaviour. | ||
Attachment and proliferation
Attachment and proliferation was determined at days 1, 7, 14, and 28 via MTS assay. Throughout the entire culture period, there is an increase at each time point and significant differences between day 14 and day 28 for all scaffolds and TCP (Fig. 5).Gene expression via RT-PCR
RT-PCR (n = 5) was used to determine the differentiation capability when the BMSCs were seeded onto each scaffold. Each scaffold, plain or complete, was split at the cartilage bone junction before RNA extraction. The gel electrophoresis (Fig. 6) shows positive expression of housekeeping gene, GAPDH, in all samples, BMP-2, Type I collagen, Type IV collagen, OPN, and Sox-9. Both the plain and complete cartilage side showed the positive expression of aggrecan, Type II collagen, and COMP. The plain and complete bone side showed positive expression of TCP, BSP, and Runx-2. It can be observed that the bands for aggrecan and Type II collagen are more intense for the cartilage side with CS and HYA and for the bone scaffold with HA, Runx-2 is much more intense visibly. Thus, the chondrogenic genes of Type II collagen, aggrecan, and COMP were all expressed in the scaffolds where cartilage is supposed to form. | ||
| Fig. 6 Gel electrophoresis of RT-PCR for the different areas of the plain and complete osteochondral scaffold. | ||
Immunofluorescence imaging for protein secretion
Visualization of the protein and its alignment was done through immunofluorescence imaging using confocal microscopy. Several different antibodies were used to probe each scaffold for BMP-2, OPN, Type I Collagen, Type II Collagen, and aggrecan. Due to the difference in physical properties of the microsphere shell and the inner spiral scaffold, the scaffold could not be cut using conventional imbedding techniques. Thus to visualize the different alignments of cells, the inner spiral scaffolds were removed from the microsphere shell and laid out carefully so as to not disturb the top horizontal layer of nanofibers.From the confocal images, it can be seen that aggrecan is expressed in both plain and complete cartilage scaffolds (Fig. 7). In both cases the bone area showed minimal secretion as characterized by the lack of green color (not shown). Both cartilage sides show alignment of the aggrecan protein in the horizontally and uniaxially aligned nanofibers. In the dually aligned images, the alignment of both directions can be seen with a clear boundary. For Type II Collagen, results (Fig. 8) were similar to that of the aggrecan images. With clear alignment that matches that of the nanofiber directions, Type II Collagen appears to be secreted more in the complete cartilage layer with CS and HYA than the plain cartilage layer lacking CS and HYA. In the 20× images (Fig. 8C–F), alignment of the protein can be seen in both the horizontally and uniaxially aligned nanofibers. The alignment and secretion of Type II Collagen and aggrecan both enforce the growth of hyaline cartilage.
For bone layers, plain and complete, there was no secretion of Type II collagen marked by the absence of green (not shown). Type I Collagen was found in all samples, plain and complete, cartilage and bone, and the alignment was matching that of the nanofibers in the cartilage layers (not shown). BMP-2 was also secreted in all samples, but visibly more in the bone layer with HA than the rest. Like the RT-PCR results, OPN was found in all samples (not shown).
Discussion
Since the 1970's, it has been known that the anisotropy of the collagenous fibrils in cartilage has provided the load-carrying capability of articular cartilage.7,15 Though there has been much research in developing scaffolds for the regeneration of cartilage, not many have been able to work as well as an autograft and be able to provide the correct cues to induce alignment.21,22,39,40 The current study focuses on designing a biomimetic polymeric scaffold that induces the regeneration of hyaline cartilage simultaneously with the subchondral bone.Similar to previous studies, aligned woven structures have been used to create an anisotropic effect for cartilage regeneration, by incorporating differently aligned nanofibers, the anisotropic properties of collagen and aggrecan protein can be induced to align them in the direction similar to the ECM of natural cartilage.41 To induce chondrogenesis or osteogenesis of the BMSCs, CS and HYA were both crosslinked via EDC/NHS coupling with the PCL in the cartilage layers and HA was blended into the bone region of the scaffold. The scaffold was able to induce the differentiation without the use of growth factors or specialized mediums. This was similar to other studies using CS and HYA, where when those materials, especially HYA was included, chondrogenesis occurred.21,22,42–46
For the bone area, it has been well known that HA has been able to induce osteogenesis and induce drastically enhance the efficiency of new bone formation when implanted into rabbits and seeded with BMSCs.47–50 Our previous studies, along with many others, have indicated that when BMSCs interact with HA combined with other biodegradable polymers, markers and proteins such as BGLAP, RUNX-2, BSP, and OPN will be expressed and secreted as when compared to scaffolds with no HA.22,50–54
Similar to the previous study, FTIR confirmed that throughout the crosslinking process CS and HYA was able to stay bound to the PCL in the cartilage region. The amine and carboxylic acid groups of CS and HYA were clearly characterized at several of the commonly found peaks including 3400, 1375, and 1310 cm−1. These results match that of our previous study that used dimethylmethylene blue assay to confirm the presence of glycosaminoglycans.21 Additionally, the HA was able to stay bound with the PCL after the various sterilization and washing techniques.
Optical microscopy showed that the scaffold was successfully fabricated using simple techniques such as mold casting, salt leeching, and electrospinning. Clear nanofiber alignment can be seen; in the cartilage region, the top layer of nanofibers are parallel to the surface movement while the underlying spiral scaffold has uniaxially aligned fibers that are perpendicular to the top fibers providing a better match to natural cartilage. Immunofluorescence imaging shows that the BMSCs seeded onto the scaffolds at day 28 clearly align themselves to that of the fiber orientation (Fig. 6 and 7). The main structural proteins of cartilage, Type II Collagen and aggrecan were confirmed by the confocal imaging. The alignment of the protein secretion itself can be seen in elongated shapes following the nanofibers. The confocal imaging also showed that the cells stay attached to the nanofibers as opposed to the underlying spiral scaffold.
The proliferation and attachment assays show that plain osteochondral scaffolds, lacking CS and HYA, seemed to promote significantly higher proliferation by day 28, as compared to complete osteochondral scaffolds with CS and HYA. This seems most likely due to the reduced differentiation of the cells, which allows them to stay in the proliferation stage.55 RT-PCR successfully confirmed the differentiation of the BMSCs seeded onto the different scaffolds. Chondrogenesis is marked by the expression of aggrecan and Type II Collagen, was mostly found in the complete cartilage layer. It is important to note that the bone scaffolds were absent of both. This shows that the scaffold structure itself is able to induce different ECM formation based on material. It can also be seen that the anisotropic surface of the plain nanofibers is also able to induce aggrecan and Type II Collagen, leading to the idea that topological surface cues may be enough to induce differentiation.15,21,22,53,56–60
Due to the location of the osteochondral tissue, it is imperative that the scaffold be elastically resistant to protect the regenerating tissue from extreme compressive conditions that can initialize damage and apoptosis of the cells. With cyclic compressive testing, it is evident that the microsphere composite scaffold is able to resist the upper limits of normal articular cartilage loading with around a 0.05% strain in uniaxial compression.
One future consideration would be to manufacture the scaffold in a more uniform method to address the concerns of the interface between the inner cartilage and bone structures as currently they are not joined together, they are only in proximity to each other.
Conclusions
Thus far the ability of the scaffold in vitro has shown that it is capable of initializing the growth of osteochondral-like tissue. With EDC/NHS water soluble CS and HYA was able to be retained within the PCL nanofibers thereby increasing the differentiation capability of the scaffold. These nanofibers were aligned to mimic the ECM orientation of natural cartilage, thereby potentially forming cartilage that is able to last longer and not require additional procedures. RT-PCR and immunofluorescence imaging confirmed both the expression, secretion, and alignment of the chondrogenic and osteogenic proteins. As testing was only performed in vitro, future steps necessitate the testing of scaffold efficacy implanted into large animals using the standard osteochondral defect model.Acknowledgements
The authors would like to thank Dr Antonio Valdevit from Stevens Institute of Technology for his assistance in the cyclic testing. Professor Yu acknowledges the funding received from the National Science Foundation IIP-1445399. Professor Kumbar acknowledges the funding from National Science Foundation IIP-1311907, IIP-1355327 and Connecticut Regenerative Medicine Research Fund-15-RMB-UCHC-08.Notes and references
- N. T. Khanarian, J. Jiang, L. Q. Wan, V. C. Mow and H. H. Lu, A Hydrogel-Mineral Composite Scaffold for Osteochondral Interface Tissue Engineering, Tissue Eng., Part A, 2011, 18(5–6), 533–545 Search PubMed.
- T. Jiang, R. R. Petersen, G. Call, G. Ofek, J. Gao and J. Q. Yao, Development of chondroitin sulfate encapsulated PLGA microsphere delivery systems with controllable multiple burst releases for treating osteoarthritis, J. Biomed. Mater. Res., Part B, 2011, 97, 355–363 CrossRef PubMed.
- L. Lu, X. Zhu, R. G. Valenzuela, B. L. Currier and M. J. Yaszemski, Biodegradable polymer scaffolds for cartilage tissue engineering, Clin. Orthop. Relat. Res., 2001, 391, S251 CrossRef PubMed.
- E. B. Hunziker, Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects, Osteoarthritis Cartilage, 2002, 10, 432–463 CrossRef CAS PubMed.
- A. M. Bhosale and J. B. Richardson, Articular cartilage: structure, injuries and review of management, Br. Med. Bull., 2008, 87, 77–95 CrossRef PubMed.
- J. Buckwalter, L. Rosenberg and E. Hunziker, Articular cartilage: composition, structure, response to injury, and methods of facilitating repair, in Articular Cartilage and Knee Joint Function: Basic Science and Arthroscopy, ed. J. Ewing, Raven Press ltd, New York, 1990, pp. 19–56 Search PubMed.
- I. C. Clarke, Articular cartilage: a review and scanning electron microscope study: 1. The interterritorial fibrillar architecture, J. Bone Jt. Surg., Br. Vol., 1971, 53, 732 CAS.
- D. Eyre, Collagen of articular cartilage, Arthritis Res., 2002, 4, 30–35 CrossRef CAS PubMed.
- K. E. Kuettner, V. A. Memoli, B. U. Pauli, N. C. Wrobel, E. Thonar and J. C. Daniel, Synthesis of cartilage matrix by mammalian chondrocytes in vitro. II. Maintenance of collagen and proteoglycan phenotype, J. Cell Biol., 1982, 93, 751–757 CrossRef CAS PubMed.
- D. Xue, Q. Zheng, C. Zong, Q. Li, H. Li and S. Qian, et al., Osteochondral repair using porous poly (lactide-co-glycolide)/nano-hydroxyapatite hybrid scaffolds with undifferentiated mesenchymal stem cells in a rat model, J. Biomed. Mater. Res., Part A, 2010, 94, 259–270 CrossRef PubMed.
- E. H. Mrosek, J. C. Schagemann, H. W. Chung, J. S. Fitzsimmons, M. J. Yaszemski and R. M. Mardones, et al., Porous tantalum and poly-ε-caprolactone biocomposites for osteochondral defect repair: Preliminary studies in rabbits, J. Orthop. Res., 2010, 28, 141–148 CAS.
- J. K. Sherwood, S. L. Riley, R. Palazzolo, S. C. Brown, D. C. Monkhouse and M. Coates, et al., A three-dimensional osteochondral composite scaffold for articular cartilage repair, Biomaterials, 2002, 23, 4739–4751 CrossRef CAS PubMed.
- A. J. Nixon, L. Begum, H. O. Mohammed, B. Huibregtse, M. M. O'Callaghan and G. L. Matthews, Autologous chondrocyte implantation drives early chondrogenesis and organized repair in extensive full- and partial-thickness cartilage defects in an equine model, J. Orthop. Res., 2011, 29, 1121–1130 CrossRef CAS PubMed.
- C. Willers, J. Chen, D. Wood, J. Xu and M. Zheng, Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits, Tissue Eng., 2005, 11, 1065–1076 CrossRef CAS PubMed.
- S. K. De Visser, J. C. Bowden, E. Wentrup-Byrne, L. Rintoul, T. Bostrom and J. M. Pope, et al., Anisotropy of collagen fibre alignment in bovine cartilage: comparison of polarised light microscopy and spatially resolved diffusion-tensor measurements1, Osteoarthritis Cartilage, 2008, 16, 689–697 CrossRef CAS PubMed.
- J. Wu and W. Herzog, Elastic anisotropy of articular cartilage is associated with the microstructures of collagen fibers and chondrocytes, J. Biomech., 2002, 35, 931–942 CrossRef CAS PubMed.
- J. M. Clark, Variation of collagen fiber alignment in a joint surface: a scanning electron microscope study of the tibial plateau in dog, rabbit, and man, J. Orthop. Res., 1991, 9, 246–257 CrossRef CAS PubMed.
- J. Wang, C. M. Valmikinathan, W. Liu, C. T. Laurencin and X. Yu, Spiral-structured, nanofibrous, 3D scaffolds for bone tissue engineering, J. Biomed. Mater. Res., Part A, 2010, 93, 753–762 Search PubMed.
- C. M. Valmikinathan, J. Tian, J. Wang and X. Yu, Novel nanofibrous spiral scaffolds for neural tissue engineering, J. Neural Eng., 2008, 5, 422 CrossRef PubMed.
- M. I. Hassan, N. Sultana and S. Hamdan, Bioactivity assessment of poly (ε-caprolactone)/hydroxyapatite electrospun fibers for bone tissue engineering application, J. Nanomater., 2014, 2014, 8 Search PubMed.
- P. Lee, K. Tran, W. Chang, N. B. Shelke, S. G. Kumbar and X. Yu, Influence of Chondroitin Sulfate and Hyaluronic Acid Presence in Nanofibers and Its Alignment on the Bone Marrow Stromal Cells: Cartilage Regeneration, J. Biomed. Nanotechnol., 2014, 10, 1469–1479 CrossRef CAS PubMed.
- P. Lee, K. Tran, G. Zhou, A. Bedi, N. B. Shelke, X. Yu and S. G. Kumbar, Guided differentiation of bone marrow stromal cells on co-cultured cartilage and bone scaffolds, Soft Matter, 2015, 11, 7648–7655 RSC.
- G. I. Im, J. H. Ahn, S. Y. Kim, B. S. Choi and S. W. Lee, A hyaluronate–atelocollagen/β-tricalcium phosphate–hydroxyapatite biphasic scaffold for the repair of osteochondral defects: A porcine study, Tissue Eng., Part A, 2010, 16, 1189–1200 CrossRef CAS PubMed.
- H. W. Kim, H. E. Kim and V. Salih, Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds, Biomaterials, 2005, 26, 5221–5230 CrossRef CAS PubMed.
- H. W. Kim, J. C. Knowles and H. E. Kim, Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery, Biomaterials, 2004, 25, 1279–1287 CrossRef CAS PubMed.
- X. Shi, Y. Wang, L. Ren, Y. Gong and D. A. Wang, Enhancing alendronate release from a novel PLGA/hydroxyapatite microspheric system for bone repairing applications, Pharm. Res., 2009, 26, 422–430 CrossRef CAS PubMed.
- H. Tsuchida, J. Hashimoto, E. Crawford, P. Manske and L. Jueren, Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats, J. Orthop. Res., 2003, 21, 44–53 CrossRef PubMed.
- J. M. Anderson and M. S. Shive, Biodegradation and biocompatibility of PLA and PLGA microspheres, Adv. Drug Delivery Rev., 1997, 28, 5–24 CrossRef CAS.
- J. L. Brown, M. S. Peach, L. S. Nair, S. G. Kumbar and C. T. Laurencin, Composite scaffolds: Bridging nanofiber and microsphere architectures to improve bioactivity of mechanically competent constructs, J. Biomed. Mater. Res., Part A, 2010, 95, 1150–1158 CrossRef PubMed.
- A. Tampieri, M. Sandri, E. Landi, D. Pressato, S. Francioli and R. Quarto, et al., Design of graded biomimetic osteochondral composite scaffolds, Biomaterials, 2008, 29, 3539–3546 CrossRef CAS PubMed.
- C. Scotti, D. Wirz, F. Wolf, D. J. Schaefer, V. Bürgin and A. U. Daniels, et al., Engineering human cell-based, functionally integrated osteochondral grafts by biological bonding of engineered cartilage tissues to bony scaffolds, Biomaterials, 2010, 31, 2252–2259 CrossRef CAS PubMed.
- A. A. Dhollander, K. Liekens, K. F. Almqvist, R. Verdonk, S. Lambrecht and D. Elewaut, et al., A pilot study of the use of an osteochondral scaffold plug for cartilage repair in the knee and how to deal with early clinical failures, Arthroscopy: The Journal of Arthroscopic & Related Surgery, 2012, 28, 225–233 Search PubMed.
- P. Lee, K. Tran, W. Chang, Y.-L. Fang, G. Zhou and R. Junka, et al., Bioactive polymeric scaffolds for osteochondral tissue engineering: in vitro evaluation of the effect of culture media on bone marrow stromal cells, Polym. Adv. Technol., 2015, 26, 1476–1485 CrossRef CAS.
- R. James, U. Toti, C. Laurencin and S. Kumbar, Electrospun Nanofibrous Scaffolds for Engineering Soft Connective Tissues, in Biomedical Nanotechnology, ed. S. J. Hurst, Humana Press, 2011. pp. 243–258 Search PubMed.
- S. Kumbar, R. James, S. Nukavarapu and C. Laurencin, Electrospun nanofiber scaffolds: engineering soft tissues, Biomed. Mater., 2008, 3, 034002 CrossRef CAS PubMed.
- J. Wang and X. Yu, Preparation, characterization and in vitro analysis of novel structured nanofibrous scaffolds for bone tissue engineering, Acta Biomater., 2010, 6, 3004–3012 CrossRef CAS PubMed.
- R. L. Mauck, M. A. Soltz, C. C. B. Wang, D. D. Wong, P. H. G. Chao and W. B. Valhmu, et al., Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels, J. Biomech. Eng., 2000, 122, 252 CrossRef CAS PubMed.
- X. Li, Y. Zhang and G. Qi, Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal stem cells, Cytotechnology, 2013, 65, 323–334 CrossRef CAS PubMed.
- S. P. Nukavarapu and D. L. Dorcemus, Osteochondral tissue engineering: Current strategies and challenges, Biotechnol. Adv., 2013, 31, 706–721 CrossRef CAS PubMed.
- D. Puppi, F. Chiellini, A. M. Piras and E. Chiellini, Polymeric materials for bone and cartilage repair, Prog. Polym. Sci., 2010, 35, 403–440 CrossRef CAS.
- Z. Ge, C. Li, B. C. Heng, G. Cao and Z. Yang, Functional biomaterials for cartilage regeneration, J. Biomed. Mater. Res., Part A, 2012, 100, 2526–2536 Search PubMed.
- M. Akmal, A. Singh, A. Anand, A. Kesani, N. Aslam and A. Goodship, et al., The effects of hyaluronic acid on articular chondrocytes, J. Bone Jt. Surg., Br. Vol., 2005, 87, 1143–1149 CrossRef CAS PubMed.
- I. L. Kim, R. L. Mauck and J. A. Burdick, Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid, Biomaterials, 2011, 32(34), 8771–8782 CrossRef CAS PubMed.
- S. Yamane, N. Iwasaki, T. Majima, T. Funakoshi, T. Masuko and K. Harada, et al., Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering, Biomaterials, 2005, 26, 611–619 CrossRef CAS PubMed.
- F. Zhang, C. He, L. Cao, W. Feng, H. Wang and X. Mo, et al., Fabrication of gelatin-hyaluronic acid hybrid scaffolds with tunable porous structures for soft tissue engineering, Int. J. Biol. Macromol., 2011, 474–481 CrossRef CAS PubMed.
- L. Zhang, K. Li, W. Xiao, L. Zheng, Y. Xiao, H. Fan and X. Zhang, Preparation of collagen-chondroitin sulfate-hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro, Carbohydr. Polym., 2010, 84(1), 118–125 CrossRef.
- J. Huang, Y. W. Lin, X. W. Fu, S. M. Best, R. A. Brooks and N. Rushton, et al., Development of nano-sized hydroxyapatite reinforced composites for tissue engineering scaffolds, J. Mater. Sci.: Mater. Med., 2007, 18, 2151–2157 CrossRef CAS PubMed.
- H. Wang, Y. Li, Y. Zuo, J. Li, S. Ma and L. Cheng, Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering, Biomaterials, 2007, 28, 3338–3348 CrossRef CAS PubMed.
- J. Venugopal, S. Low, A. T. Choon, T. Sampath Kumar and S. Ramakrishna, Mineralization of osteoblasts with electrospun collagen/hydroxyapatite nanofibers, J. Mater. Sci.: Mater. Med., 2008, 19, 2039–2046 CrossRef CAS PubMed.
- B.-S. Kim, J. S. Kim, Y. S. Chung, Y.-W. Sin, K.-H. Ryu, J. Lee and H. K. You, Growth and osteogenic differentiation of alveolar human bone marrow-derived mesenchymal stem cells on chitosan/hydroxyapatite composite fabric, J. Biomed. Mater. Res., Part A, 2012, 101(6), 1550–1558 Search PubMed.
- B. Chuenjitkuntaworn, W. Inrung, D. Damrongsri, K. Mekaapiruk, P. Supaphol and P. Pavasant, Polycaprolactone/hydroxyapatite composite scaffolds: Preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells, J. Biomed. Mater. Res., Part A, 2010, 94, 241–251 CrossRef PubMed.
- Y. X. Gu, J. Du, J. M. Zhao, M. S. Si, J. J. Mo and H. C. Lai, Characterization and preosteoblastic behavior of hydroxyapatite-deposited nanotube surface of titanium prepared by anodization coupled with alternative immersion method, J. Biomed. Mater. Res., Part B, 2012, 100(8), 2122–2130 CrossRef PubMed.
- A. Ergun, X. Yu, A. Valdevit, A. Ritter and D. M. Kalyon, Radially and Axially Graded Multizonal Bone Graft Substitutes Targeting Critical-Sized Bone Defects from Polycaprolactone/Hydroxyapatite/Tricalcium Phosphate, Tissue Eng., Part A, 2012, 18, 2426–2436 CrossRef CAS PubMed.
- X. Zhang, W. Chang, P. Lee, Y. Wang, M. Yang and J. Li, et al., Polymer-Ceramic Spiral Structured Scaffolds for Bone Tissue Engineering: Effect of Hydroxyapatite Composition on Human Fetal Osteoblasts, PLoS One, 2014, 9, e85871 Search PubMed.
- S. B. Rønning, M. E. Pedersen, P. V. Andersen and K. Hollung, The combination of glycosaminoglycans and fibrous proteins improves cell proliferation and early differentiation of bovine primary skeletal muscle cells, Differentiation, 2013, 86, 13–22 CrossRef PubMed.
- W.-J. Li, R. L. Mauck, J. A. Cooper, X. Yuan and R. S. Tuan, Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering, J. Biomech., 2007, 40, 1686–1693 CrossRef PubMed.
- B. M. Baker and R. L. Mauck, The effect of nanofiber alignment on the maturation of engineered meniscus constructs, Biomaterials, 2007, 28, 1967–1977 CrossRef CAS PubMed.
- B. Zhu, Q. Lu, J. Yin, J. Hu and Z. Wang, Alignment of osteoblast-like cells and cell-produced collagen matrix induced by nanogrooves, Tissue Eng., 2005, 11, 825–834 CrossRef CAS PubMed.
- C. H. Lee, H. J. Shin, I. H. Cho, Y. M. Kang, I. Kim and K. D. Park, et al., Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast, Biomaterials, 2005, 26, 1261–1270 CrossRef CAS PubMed.
- A. Ergun, X. Yu, A. Valdevit, A. Ritter and D. M. Kalyon, In vitro analysis and mechanical properties of twin screw extruded single-layered and coextruded multilayered poly (caprolactone) scaffolds seeded with human fetal osteoblasts for bone tissue engineering, J. Biomed. Mater. Res., Part A, 2011, 99(3), 354–366 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |