Thermoresponsive dynamic covalent dendronized polymers†
Abstract
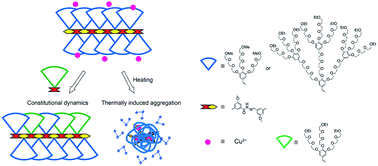
Thermoresponsive dendronized polymers constituted with a polyacylhydrazone backbone pendanted with oligoethylene glycol (OEG)-based dendrons were synthesized through polycondensation of dialdehydes and diacylhydrazines. First (G1) and second (G2) generation OEG dendrons were selected for comparison to examine the dendritic effects. Inherited from OEG dendrons, these polymers showed characteristic thermoresponsive behavior. By virtue of the dynamic property of the acylhydrazone linkage, formation of these polymers are concentration and pH-dependent. A dimer from monoaldehyde and monoacylhydrazine was prepared as a model to support the remarkable macromolecular effects for enhanced structural stability of polyacylhydrazones. Constitutional stability of these polyacylhydrazones were also testified by switching solutions to strong acidic or basic conditions. The structural vibration was simply testified by addition of a new monomer as a competitor to dendronized polyacylhydrazones, thus, resulting in polymers with tunable phase transition temperatures. Furthermore, G1 dendrons were found to provide efficient shielding for acylhydrazones from an affinity to metal ions, but this shielding effect diminished and exhibited specific interactions with Cu2+ when the dendron collapsed.

 Please wait while we load your content...
Please wait while we load your content...