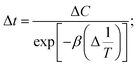DOI:
10.1039/C6RA08216E
(Paper)
RSC Adv., 2016,
6, 46616-46624
Kinetic analysis of Fe3+ reduction coupled with nitrate removal by Klebsiella sp. FC61 under different conditions
Received
30th March 2016
, Accepted 4th May 2016
First published on 6th May 2016
Abstract
Klebsiella sp. FC61, a newly found iron-reducing bacterium, has the ability of simultaneously reducing Fe3+ and nitrate under different pH and temperature conditions. Based on kinetic equations, the maximum rate of Fe3+ reduction was 0.26 mg L−1 h−1 at pH 6.0 and 0.24 mg L−1 h−1 at 30 °C. The maximum rate of nitrate removal was 0.21 mg L−1 h−1 at pH 7.0 and 0.82 mg L−1 h−1 at 35 °C. After 56 h, nitrate was removed completely, coupled with Fe3+ reduction, and there was a transient accumulation of nitrite. Fe3+ and nitrate reduction followed the selected kinetic equations well. Moreover, the Arrhenius equation further confirmed the reliability of the data under the different temperature conditions. When analyzed using XRD and SEM, a form of crystalline iron oxide with recovered deposits of strain FC61 was observed.
1. Introduction
Nitrate, a widespread contaminant in drinking water, when ingested under conditions resulting in endogenous nitrosation, is suspected to be carcinogenic.1 The organisms capable of coupling Fe3+ reduction to nitrate removal can be referred to as anaerobic Fe3+-reduction denitrifiers; this process is relatively rare in the biological world.2 Observations of Fe3+-reducing bacteria on the roots of wetland plants3 suggest that Fe2+ oxidation and Fe3+ reduction are coupled within the rhizosphere, promoting a localized iron cycle. It has been suggested that these bacteria can also be used in groundwater to remove nitrate. However, very few studies have been carried out to analyze the dynamics of the reaction. Reduction of Fe3+ is closely related to bacterial growth and in many cases significant anaerobic growth does not occur in the absence of Fe3+.4 The study of iron and nitrogen cycling system shows that the iron reducing bacteria can convert Fe3+ to Fe2+, in turn, Fe2+ which has been turned can become an electron donor for denitrification to convert Fe2+ to Fe3+ at the same time. Therefore, the process forms an iron cycle. The process can be simply described as: Fe3+ + NO3− + H2O + H+ → Fe2+ + N2 + H2O (or) Fe2+ + N2 + NO2− + H2O.5,6 Monod-type growth kinetics is most often used to describe experimental observations for biological substrate degradation processes. This model describes the degradation of nitrogen, and is a suitable illustration of the use of Monod growth kinetics to describe degradation processes.7 The model describes biomass concentration (x) as a function of time (t), based on logistic equation, and establishes the accumulation of microbial material with varying time. This work is helpful for studying microbial growth, and the decomposition process of degradable substances by microorganisms in an water environment.8,9 A method was developed to reliably analyze the growth kinetics data taken from different stress environments, as proposed by the Arrhenius stress model. The parameters in the model were estimated by the maximum likelihood method.10 Currently, there have been many studies on bacterial biological mineralization; however, only a few bacteria exhibit this ability.11 Different species of bacteria have different optimum pH and temperature; there is no definite effective pH and temperature range for all strains. Importantly, pH and temperature are key factors for the growth of microorganisms,12 as shown in the present study on denitrification that pH for optimal bacterial growth ranged from 6.0 to 9.0 and for the iron-reducing process the temperature range was 25–45 °C.13,14 Therefore, we chose different pH and temperature conditions to study the strain FC61 in this investigation.
In present study, we study the characterization of Klebsiella sp. FC61 with ability of reducing Fe3+ and nitrate under different pH and temperature. Moreover, Fe3+ and nitrate reduction kinetics and the mineralogy of Fe3+ reduction produced were also given an illustration and it would provide a basis for the later data prediction through the simulation of the data.
2. Materials and methods
2.1. Bacterial strain and media
The strain FC61 was isolated from a sludge sample which was collected from Tang Yu oligotrophic reservoir (Shanxi Province, China). And the strain was identified as Klebsiella sp. based on 16S rRNA gene sequence and our previous data analysis, the GenBank accession number of strain FC61 was KT860061.15 In this investigation, the inoculation dose was 5% (v/v). All chemical reagents used were analytical grade without further purification. Solutions used in this experiment were prepared using ultra-pure water that was produced using a Milli-Q device (Millipore, USA). The media used in this investigation comprised the following reagents (g L−1): NaHCO3, 0.5; NaNO3, 0.12; KH2PO4, 0.05; Fe2(SO4)3, 0.11; MgSO4·7H2O, 0.05; CaCl2, 0.05; and trace element solution, 2 mL. The components of trace element solution for the bacteria growth was as follows: MgSO4·7H2O 0.5 (g L−1), EDTA 1 (g L−1), ZnSO4 0.2 (g L−1), MnCl2·4H2O 0.1 (g L−1), FeSO4·7H2O 0.15 (g L−1), CuSO4·5H2O 0.5 (g L−1), CoCl·6H2O 0.2 (g L−1). All of the mediums were prepared by heating the solution to 121 °C in 30 min and immediately cooling it to room temperature under an anoxic atmosphere.
2.2. Analytical methods of experimental data
Fe(II) concentration was measured spectrophotometrically with phenanthroline at 510 nm. Nitrate-N concentration was determined by an UV spectrophotometric screening method by calculating the difference between OD220 and 2 × OD275. Nitrite-N concentration was determined by colourimetry at wavelengths of 540 nm using N-(1-naphthyl)-1,2-diaminoethane dihydrochloride method. The biomass was measured by means of nephelometry,16 and the corresponding absorption value was at a wavelength of 600 nm. The pH (HQ11d, HACH, USA) of the medium was adjusted by 1 mol L−1 HCl or NaOH solution and ultra-pure water were used in this study. The temperature was controlled by the constant temperature incubator.
2.3. Analytical methods of dynamics
To evaluate effects of different pH and temperature on the accumulation and optimal incubation time of Fe2+, that the kinetic models based on a logistic equation,17,18 the formula of| |
 | (1) |
was used in this study. S(t) was the concentration of Fe2+ (mg L−1) which was measured at time t (h). The parameters of this formula were a (the largest limit factor of S(t)), b (the intercept of y-axis) and c (the reaction velocity constant for Fe2+ accumulation). And the software Curve Expert 1.3 was used to estimate the parameters. Meanwhile, to evaluate effects of different pH and temperature on the reduction of nitrate, that the kinetic models based on the Monod equation, the formula of19| |
 | (2) |
was used in this study. V was the degradation rate of nitrate (mg L−1 h−1). ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) was the average concentration of microorganisms (mg L−1). C(t) was the concentration of nitrate (mg L−1) which was measured at time t (h). Moreover, in order to evaluate the reliability of the data at different temperatures, Arrhenius equation:20
was the average concentration of microorganisms (mg L−1). C(t) was the concentration of nitrate (mg L−1) which was measured at time t (h). Moreover, in order to evaluate the reliability of the data at different temperatures, Arrhenius equation:20| |
 | (3) |
was used, C(t) was the concentration of nitrate (mg L−1) which was measured at time t (h), α, β was the constant of Arrhenius equation. T0, the selected base temperature, intermediate temperature of 27.5 °C was selected in this investigation. Tn was the experimental controlled temperature: 20 °C, 25 °C, 30 °C, 35 °C.
2.4. Analytical methods of the sediment
The sediment of strain FC61 was analyzed by a scanning electron microscopy (SEM) (JSM-5800, Japan JEOL). The composition and degree of crystallinity of the sediment was analyzed by means of X-ray diffraction (XRD). The samples were dehydrated using a series of different concentrations of ethanol (40%, 60%, 80%, 90%, 100%) followed by drying.
3. Results and discussion
3.1. Estimation of parameters of logistic and Monod equation
The accumulation of Fe2+ concentration rate V could obtained through| |
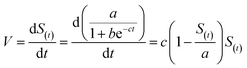 | (4) |
(The meaning of the specific parameters had been listed in 2.3). In order to estimate the relevant parameters, the following deduction could be done:| |
 | (5) |
and when made| |
 | (6) |
| |
 | (7) |
it could be obtained the relevant estimation of parameters, the maximum accumulation rate of Fe2+ was Vmax = 0.25ac, which could be calculated through putting S(t) = a/2 into eqn (4), and the corresponding time was Tmax = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/c. The data were presented in Table 1 through curves fitting by using the software Curve Expert 1.3. Meanwhile, the degradation rate of nitrate could be obtained and from the following deduction:
b/c. The data were presented in Table 1 through curves fitting by using the software Curve Expert 1.3. Meanwhile, the degradation rate of nitrate could be obtained and from the following deduction:| |
 | (8) |
![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) was the average concentration of nitrate between t and t − 1 (t ≥ 1), K was the saturation constant when V = 0.5Vmax. The formula was regarded as the linear equation of y = nx + m, V−1 = y, x =
was the average concentration of nitrate between t and t − 1 (t ≥ 1), K was the saturation constant when V = 0.5Vmax. The formula was regarded as the linear equation of y = nx + m, V−1 = y, x = ![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) −1, K/Vmax = n, Vmax−1 = m, therefore, Vmax = 1/m and K = n/m could be obtained by linear fitting, m was the intercept of y-axis and n was the slope. Moreover, the kinetics parameters of nitrate removal were presented in Table 2. The Adj. R-square showed that the data more closer to 1, the better the correlation. And Pearson's r was also used as a test of significance for the data, when the Pearson's r was close to 1, showed that the data was relatively significant.21 Therefore, it could be indicated that the data were in agreement with the selected kinetic equation well as the r or R were close to 1 in this study.
−1, K/Vmax = n, Vmax−1 = m, therefore, Vmax = 1/m and K = n/m could be obtained by linear fitting, m was the intercept of y-axis and n was the slope. Moreover, the kinetics parameters of nitrate removal were presented in Table 2. The Adj. R-square showed that the data more closer to 1, the better the correlation. And Pearson's r was also used as a test of significance for the data, when the Pearson's r was close to 1, showed that the data was relatively significant.21 Therefore, it could be indicated that the data were in agreement with the selected kinetic equation well as the r or R were close to 1 in this study.
Table 1 Kinetics parameters of Fe3+ reduction under different pH and temperaturesa
| Factors |
Model parameters |
Vmax [mg L−1 h−1] |
Tmax [h] |
| a |
b |
c |
s |
r |
| a, the largest limit factor of Fe2+ concentration; b, the intercept of y-axis; c, the reaction velocity constant for Fe2+ accumulation; s, the standard error estimate in the regression models; r, the correlation coefficient; Vmax, the maximum reduction rate of Fe3+; Tmax the time when the reduction rate of Fe3+ reaches Vmax. |
| pH of 5 |
11.37 |
16.62 |
0.06 |
0.7684 |
0.9779 |
0.16 |
48.84 |
| pH of 6 |
5.13 |
15.7 |
0.20 |
0.5153 |
0.9749 |
0.26 |
13.77 |
| pH of 7 |
7.29 |
14.19 |
0.10 |
0.6150 |
0.9816 |
0.19 |
26.53 |
| pH of 8 |
8.17 |
8.39 |
0.08 |
0.8858 |
0.9590 |
0.16 |
26.59 |
| Temperature of 20 °C |
4.67 |
88.19 |
0.10 |
0.2112 |
0.9944 |
0.12 |
44.79 |
| Temperature of 25 °C |
6.87 |
14.33 |
0.11 |
0.6244 |
0.9790 |
0.19 |
24.20 |
| Temperature of 30 °C |
6.07 |
15.71 |
0.16 |
0.5089 |
0.9827 |
0.24 |
17.21 |
| Temperature of 35 °C |
6.11 |
13.75 |
0.15 |
0.4981 |
0.9837 |
0.22 |
17.47 |
Table 2 Kinetics parameters of nitrate removal under different pH and temperaturesa
| Factors |
Regression equations |
Pearson's r |
Adj. R-square |
KN [mg L−1] |
Vmax [mg L−1 h−1] |
| KN, the saturation constant; Vmax, the maximum removal rate of nitrate. |
| pH of 5 |
y = 60.6648x + 5.8056 |
0.9997 |
0.9994 |
11.48 |
0.17 |
| pH of 6 |
y = 68.1744x + 15.8433 |
0.9643 |
0.9123 |
4.30 |
0.06 |
| pH of 7 |
y = 59.9457x + 4.8657 |
0.9873 |
0.9966 |
12.32 |
0.21 |
| pH of 8 |
y = 47.2731x + 4.9461 |
0.9869 |
0.9634 |
9.56 |
0.20 |
| Temperature of 20 °C |
y = 64.9362x + 10.9518 |
0.9996 |
0.9991 |
5.93 |
0.09 |
| Temperature of 25 °C |
y = 45.1380x + 7.1271 |
0.9885 |
0.9725 |
6.33 |
0.14 |
| Temperature of 30 °C |
y = 26.3770x + 2.1247 |
0.9995 |
0.9987 |
12.41 |
0.47 |
| Temperature of 35 °C |
y = 26.1577x + 1.2134 |
0.9997 |
0.9993 |
21.56 |
0.82 |
| Linear fitting of Arrhenius |
y = 46.4078x − 0.7020 |
0.9506 |
0.8539 |
— |
— |
3.2. Effects of different pH on the growth of strain FC61
In this study the pH was varied, keeping the temperature constant at 30 °C in the incubator. As it shown in Fig. 1, the biomass of strain FC61 reached a maximum value of 195.84 mg L−1 at 56 h at pH 7 (pH 5, 125.51 mg L−1; pH 6, 191.93 mg L−1; pH 8, 135.77 mg L−1). Concurrently, the concentration of nitrate showed a declining trend, and at 40 h, the concentration of nitrate was 2.34 mg L−1 (pH 5), 1.02 mg L−1 (pH 6), 0 mg L−1 (pH 7), and 1.33 mg L−1 (pH 8). Compared to pH 6, 7, and 8, it could be seen that the concentration of Fe2+ was stable at pH 5 without any fluctuation, which might be due to the acidic conditions where hydrolysis of Fe2+ could be inhibited to a certain extent.22 Meanwhile, there was low accumulation of nitrite during the experiment, and the nitrite concentration at 8 h reached a maximum level of 0.61 mg L−1 (pH 5), 0.42 mg L−1 (pH 6), 0.16 mg L−1 (pH 7), and 0.32 mg L−1 at 32 h (pH 8). After 56 h, there was almost no nitrite in the media. According to the kinetic models of logistic and Monod equation analysis (Fig. 3A–H), the maximum accumulation rate of Fe2+ under various pH conditions were as follows: pH 5, 0.16 mg L−1 h−1; pH 6, 0.26 mg L−1 h−1; pH 7, 0.19 mg L−1 h−1; and pH 8, 0.16 mg L−1 h−1. This was coupled with the maximum degradation rate of nitrate: pH 5, 0.17 mg L−1 h−1; pH 6, 0.06 mg L−1 h−1; pH 7, 0.21 mg L−1 h−1; and pH 8, 0.20 mg L−1 h−1. It could also be seen that under different pH conditions, the time to reach the maximum Fe3+ reduction rate was very short, ranging from 13.77 h to 48.84 h, and 0.5 Vmax corresponded to the saturation constant of 4.3–12.32 mg L−1 (Tables 1 and 2). It was observed that at pH 6, the Fe3+ reduction rate reached 0.26 mg L−1 h−1, which was greater than that at other pH conditions. However, the nitrate removal rate was only 0.06 mg L−1 h−1, which was the least value. It could be concluded from the data that for the strain FC61, its ability to reduce Fe3+ and degrade nitrate was in a competitive relationship when the conditions were favorable. However, concomitant with the reduction of Fe3+, the degradation of nitrate might be hampered slightly.23 Therefore, the optimal pH for strain FC61 to reduce Fe3+ and degrade nitrate was 6 and 7; taking both the pH conditions into consideration, the optimal pH for strain FC61 was 6.
 |
| | Fig. 1 Performance of strain FC61 under different pH: (A) pH of 5; (B) pH of 6; (C) pH of 7; (D) pH of 8. ■ Fe2+; ● NO3−–N; ○ biomass; ▨ NO2−–N. | |
3.3. Effects of different temperatures on the growth of strain FC61
Previous studies have indicated that bacteria were sensitive to temperature variation. For example, Zaitsev et al. indicated that temperature would affect the denitrification rate with every 4 °C increase; whereas, Ge et al. indicated that temperature would affect the activity of bacterial enzymes.24,25 In the present study, the initial temperature of the medium was set at 20 °C, 25 °C, 30 °C and 35 °C, respectively. As shown in Fig. 2, the biomass of strain FC61 reached a maximum level of 196.32 mg L−1 at 30 °C (at 20 °C, 160.19 mg L−1; 25 °C, 114.77 mg L−1; 35 °C, 184.60 mg L−1). The results showed that the biomass of strain FC61 at 25 °C was lower than at other temperature groups, which might be due to accumulation of nitrite that has a toxic effect on the growth of microorganisms, from the point of nitrite enzyme activity.26,27 The concentration of Fe2+ reached a maximum level of 4.52 mg L−1 at 64 h, 7.18 mg L−1 at 56 h, 6.59 mg L−1 at 48 h, 6.71 mg L−1 at 56 h in the temperature of 20 °C, 25 °C, 30 °C and 35 °C with a continuous rising trend. It was evident that with change in temperature, all the indices changed appreciably, in accordance with the kinetic models of logistic and Monod equation analysis (Fig. 3A–H); a deeper relationship will be sought. As shown in Table 1 the maximum accumulation rate of Fe2+ was 0.12 mg L−1 h−1, 0.19 mg L−1 h−1, 0.24 mg L−1 h−1, and 0.22 mg L−1 h−1 from 20 °C to 35 °C, respectively. Moreover, Table 2 shows that the maximum degradation rate of nitrate was 0.09 mg L−1 h−1, 0.14 mg L−1 h−1, 0.47 mg L−1 h−1, and 0.82 mg L−1 h−1 from 20 °C to 35 °C, respectively. It is also evident that at different temperatures, the time to reach the maximum Fe3+ reduction rate was 17.21 h to 44.79 h, and 0.5Vmax corresponded to the saturation constant of 5.93 mg L−1 to 21.56 mg L−1 (Tables 1 and 2). There was low accumulation of nitrite under different temperatures. As shown in Fig. 2A, before 32 h the accumulation of nitrite was very little, after which it began to accumulate rapidly, and reached the maximum level of 0.17 mg L−1 at 42 h. It was possible that the low temperature inhibited the enzyme action for reducing NO3− to NO2−. It should be noted that nitrate reduction consists of four consecutive reduction steps, involving nitrite (NO2−), nitric oxide (NO) and nitrous oxide (N2O) as three obligatory intermediates.28 At other temperatures, reduction of nitrate, would lead to smaller accumulation of nitrite. After 56 h, there was almost no nitrite in the media (Fig. 2). The kinetic data indicated that the reduction rate of Fe3+ was maximal at 30 °C. The reduction rate was slightly decreased at 35 °C, as iron reductase might be inhibited by higher temperature and since effective iron reduction required an appropriate temperature.29,30 The effect of temperature on the removal of nitrate was significantly higher than the effects on the reduction of Fe3+ from 20–30 °C; the higher the temperature, the maximal removal rate of nitrate. Importantly, in this case also, a relatively high temperature could increase the enzyme activity of denitrification.31 Therefore, the optimal temperature for strain FC61 to reduce Fe3+ and remove nitrate was 30 °C and 35 °C; taking both these temperatures into consideration, the optimal temperature for strain FC61 was 30 °C.
 |
| | Fig. 2 Performance of strain FC61 under different temperatures: (A) temperature of 20 °C; (B) temperature of 25 °C; (C) temperature of 30 °C; (D) temperature of 35 °C. ■ Fe2+; ● NO3−–N; ○ biomass; ▨ NO2−–N. | |
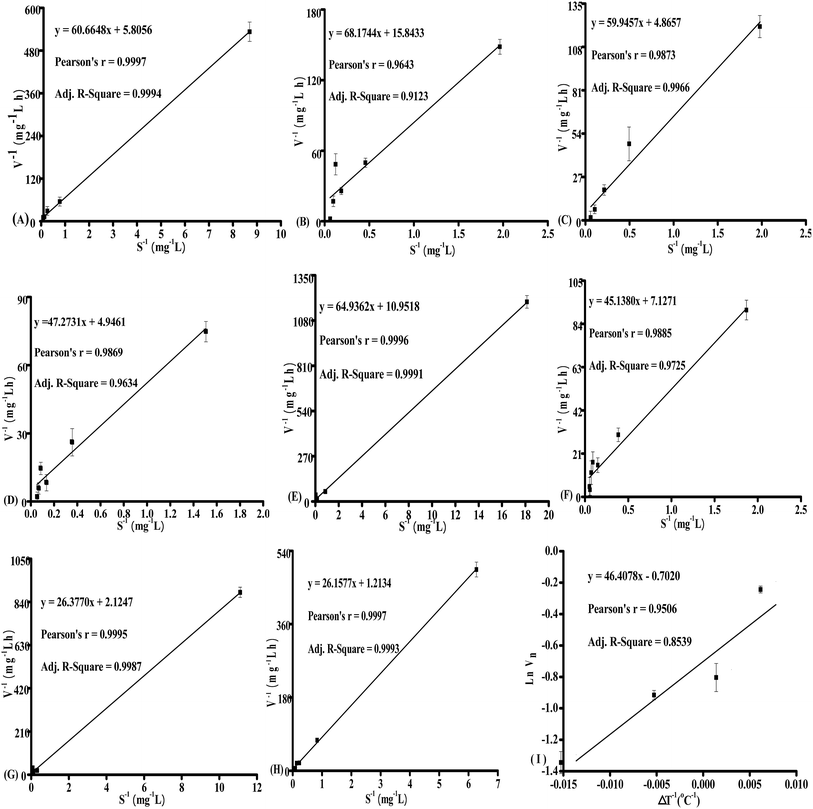 |
| | Fig. 3 The kinetic curves of NO3−–N under different pH: (A) 5; (B) 6; (C) 7; (D) 8, and different temperature: (E) 20 °C; (F) 25 °C; (G) 30 °C; (H) 35 °C, and (I) the curve of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vn to ΔT−1 from 20 °C to 35 °C based on Arrhenius equation. Vn to ΔT−1 from 20 °C to 35 °C based on Arrhenius equation. | |
3.4. Statistical analysis of reliability growth data under different temperatures
At one state of the removal nitrate was C1 (mg L−1) and the corresponding time was t1 (h); another state of the removal nitrate was C2 (mg L−1), the corresponding time was t2 (h). When ΔT−1 (°C−1) was a constant, the cumulative amount of removal nitrate from t1 to t2 was| |
 | (9) |
Therefore, ΔC(C2 − C1) could be obtained from eqn (9)
| |
 | (10) |
Further more,
| |
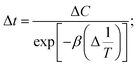 | (11) |
when the amount of removal nitrate reached a value
Cs, it is considered that the reaction was over, at this time it would affect the correct assessment of strain FC61 to remove nitrate. And the time difference (Δ
t) could be used to indicate the characteristics of nitrate degradation (
V). Therefore, it could be obtained:
| |
 | (12) |
Meanwhile, the ratio of degradation characteristics under the condition of transformation (γ = V0/V2) also could be obtained, which γ was called degradation factor in this study.32,33 When t → 0, the amount of degradation at different temperatures could be regarded as the same value Q. Therefore,
| |
 | (13) |
Finally, the degradation factor under the Arrhenius model were
| |
 | (14) |
and
| |
 | (15) |
Vn was the nitrate removal rate under different temperature based on Monod equation. The data fitting based on Arrhenius equation was shown in
Fig. 3I. From the results of the simulation, the data (Adj.
R-square was 0.8539 and Pearson's
r was 0.9506) were basically in accordance with the Arrhenius formula. Moreover, the constant of Arrhenius equation
β was 46.4078, and
V = exp[46.4078 × (0.03636 −
T−1)]
Vn.
3.5. Characteristics of biological reduction of Fe3+
Hydrolysis of Fe2(SO4)3 produces a precipitate at the beginning of the reaction (0 h); taking this as the initial time-point, and following up to the end of the reaction, the characteristics of the experimental and control groups were observed by SEM. As shown in Fig. 4A and C, at the beginning of the reaction, it could be seen that the experimental group and the control group had no significant difference with the amplification of 4500. Only dense compacted granular sediment was observed, which could be Fe(OH)3 according to the equation: Fe3+ + 3H2O ↔ Fe(OH)3 + 3H+.34 At the end of the reaction (80 h), the sediments were observed again by SEM. From Fig. 4B and D, the differences could be seen clearly. Compared to Fig. 4C, the change was not obvious at the end of the control group (Fig. 4D); just the state of compaction had been strengthened at 80 h, which might be due to the complete hydrolysis of Fe3+. However, the sediment had been changed in the experimental group (Fig. 4B). Crystalline solid was observed which was completely different from the initial state. In order to further determine the sediment composition, X-ray diffraction was used to observe the sediments of pretreatment. As shown in Fig. 5A, there was relatively strong peaks in the experimental group, which showed a crystal form. The elements Fe and O were limited, compared with the standard spectra. The software X'pert Highscore plus 3.0 indicated that the optimal match was Fe3O4.35 In contrast, the peaks of control group were irregular (Fig. 5B), and could not display a crystal structure.36 Moreover, for the general mineralization, the adsorption of organic matter and metal chelate were obvious. However, the adsorption degree of small molecule ions such as NO3−, Fe3+ and Fe2+ were weak; even P25–Ti2O with a nano-pore structure, the adsorption of Fe3+ was only 248 μg mg−1, and most of the adsorption of ions was on the mineral surface, which could be used by the underlying bacteria.37,38 The experiment was carried out using a blank control as a benchmark. The results showed that Fe2+ was produced as a result of the reaction, which further verified that the strain FC61 has the ability of Fe3+ reduction, and was probably also equipped with the ability of biological mineralization.
 |
| | Fig. 4 The change of the sediment in the experimental group (A) observed at 0 h, (B) observed at 80 h; and in the control group (C) observed at 0 h, (D) observed at 80 h, which were presented by the scanning electron microscopy. | |
 |
| | Fig. 5 Profiles of the treated sediment in (A) the experimental group; (B) the control group, which were measured at the end of the experiment by using X-ray diffraction spectrum. | |
4. Conclusions
This study reported a new discovery of iron reducing bacterium Klebsiella sp. FC61 with the ability of reducing Fe3+ and nitrate under different pH and temperature. The r or R were close to 1 in this study showed that the selected kinetic equations of logistic, Monod and Arrhenius were in agreement with the experimental data well. Moreover, logistic equation showed that the optimal pH and temperature for Fe3+ reduction were pH of 6 and temperature of 30 °C with the reduction rate of 0.26 mg L−1 h−1 and 0.24 mg L−1 h−1, and Monod equation showed that the optimal pH and temperature for nitrate removal were pH of 7 and temperature of 35 °C with the nitrate removal rate of 0.21 mg L−1 h−1 and 0.82 mg L−1 h−1. Deduction of the basic equation of Arrhenius, the data at different temperatures were reliable. Moreover, a form of crystalline iron oxide with recovered deposits of strain FC61 was observed by using XRD and SEM, which further verified the strain FC61 had the ability of Fe3+ reduction as well as biological mineralization.
Acknowledgements
This research work was partly supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People's Republic China (No. 2012BAC04B02). Supported by Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (No. QA201518).
References
- N. Espejo-Herrera, K. P. Cantor, N. Malats, D. T. Silverman, A. Tardón, R. García-Closas, C. Serra, M. Kogevinas and C. M. Villanueva, Nitrate in drinking water and bladder cancer risk in Spain, Environ. Res., 2015, 137, 299–307 CrossRef CAS PubMed.
- J. T. Zhang, J. R. Ni and S. G. Zhou, Progress in research of microbial fuel cells based on Fe(III)-reducing bacteria, Chin. J. Appl. Environ. Biol., 2008, 4, 290–295 Search PubMed.
- G. M. King and M. A. Garey, Ferric iron reduction by bacteria associated with the roots of freshwater and marine macrophytes, Appl. Environ. Microbiol., 1999, 65, 4393–4398 CAS.
- H. A. Videla and L. K. Herrera, Understanding microbiol corrosion inhibition. A comprehensive overview, Int. Biodeterior. Biodegrad., 2009, 63, 896–900 CrossRef CAS.
- V. W. Johanna, E. David, M. S. Backer and J. P. Megonigal, Enumeration of Fe(II)-oxidizing and Fe(III)-reducing bacteria in the root zone of wetland plants: implications for a rhizosphere iron cycle, Biogeochemistry, 2003, 64, 77–96 CrossRef.
- J. L. Nielsen, S. Juretschko and M. Wagner, Abundance and phylogenetic affiliation of iron reducers in activated sludge as assessed by fluorescence in situ hybridization and microautoradiography, Appl. Environ. Microbiol., 2002, 68, 4629–4636 CrossRef CAS PubMed.
- B. Petersena, K. Gernaey, M. Devisschera, D. Dochainc and P. A. Vanrolleghema, A simplified method to assess structurally identifiable parameters in Monod-based activated sludge models, Water Res., 2003, 37, 2893–2904 CrossRef.
- T. E. Shehata and A. G. Marr, Effect of nutrient concentration on the growth of Escherichia coli, J. Bacteriol., 1971, 107, 210–216 CAS.
- L. Tang, G. M. Zeng, W. Sun and W. Z. Wei, The Application of Logistic Equation in Kinetics of Microbial Batch Culture, J. Hunan Univ., Nat. Sci., 2004, 31, 23–28 Search PubMed.
- B. Huang, A. Liu and G. Li, The Statistical Analysis for Reliability Growth Data in the Arrhenius Stress Model, Chin. J. Mech. Eng., 2005, 22, 807–814 Search PubMed.
- B. Li, C. Tian, D. Zhang and X. Pan, Anaerobic Nitrate-Dependent Iron(II) Oxidation by a Novel Autotrophic Bacterium, Citrobacter freundii Strain PXL1, Geomicrobiol. J., 2014, 38, 138–144 CrossRef.
- P. Elefsiniotis and D. Li, The effect of temperature and carbon source on denitrification using volatile fatty acids, Biochem. Eng. J., 2006, 28, 148–155 CrossRef CAS.
- H. J. Hong, S. J. Kim, U. G. Min, Y. J. Lee, S. G. Kim, M. Y. Jung, Y. S. Seo and S. K. Rhee, Geosporobacter ferrireducens sp. nov., an anaerobic iron-reducing bacterium isolated from an oil-contaminated site, Antonie van Leeuwenhoek, 2015, 107, 971–977 CrossRef CAS PubMed.
- H. Liu, W. Jiang, D. Wan and J. Qu, Study of a combined heterotrophic and sulfur autotrophic denitrification technology for removal of nitrate in water, J. Hazard. Mater., 2009, 169, 23–28 CrossRef CAS PubMed.
- J. F. Su, C. Cheng, T. L Huang, F. Ma, J. S Lu and S. C Shao, Novel simultaneous Fe(III) reduction and ammonium oxidation of Klebsiella sp. FC61 under the anaerobic conditions, RSC Adv., 2016, 6, 12584–12591 RSC.
- B. Yan, L. Zhao, X. Tan and J. Gao, Quick measurement of biomass by nephelometery, Tianjin Chem. Ind., 2003, 17, 45–52 Search PubMed.
- J. He and D. Qu, Dissimilatory Fe(III) reduction characteristics of paddy soil extract cultures treated with glucose or fatty acids, J. Environ. Sci., 2008, 20, 1103–1108 CrossRef CAS.
- X. Dong, Y. Zhang, J. Zhou, N. Li and M. Chen, Reduction of Fe(III) EDTA in a NOx scrubber liquor by a denitrifying bacterium and the effects of inorganic sulfur compounds on this process, Bioresour. Technol., 2012, 120, 127–132 CrossRef CAS PubMed.
- J. Monod, The growth of Bacterial Cultures, Annu. Rev. Microbiol., 1949, 3, 371–394 CrossRef CAS.
- L. H. Crow, Reliability Analysis for Complex Repairable Systems, Soc. Industrial and Applied Mathematics, Reliability and Biometry, Proceedings of Statistical Analysis of Life Length, 1974, vol. 25, pp. 248–253 Search PubMed.
- G. I. Reynoso-Cereceda, R. I. Garcia-Cabrera, N. A. Valdez-Cruz and M. A. Trujillo-Roldán, Shaken flasks by resonant acoustic mixing versus orbital mixing: mass transfer coefficient kLa characterization and Escherichia coli cultures comparison, Biochem. Eng. J., 2016, 105, 379–390 CrossRef CAS.
- J. Y. Yang, X. E. Yang, Z. L. He, T. Q. Li, J. L. Shentu and P. J. Stoffella, Effects of pH, organic acids, and inorganic ions on lead dissolution from soils, Environ. Pollut., 2006, 143, 9–15 CrossRef CAS PubMed.
- J. L. Pierre, M. Fontecave and R. R. Crichton, Chemistry for an essential biological process: the reduction of ferric iron, BioMetals, 2009, 15, 341–346 CrossRef.
- G. Zaitsev, T. Mettänen and J. Langwaldt, Removal of ammonium and nitrate from cold inorganic mine water by fixed-bed biofilm reactors, Miner. Eng., 2008, 21, 10–15 CrossRef CAS.
- J. P. Ge, R. P. Du, D. Zhao, G. Song, M. Jina and W. X. Ping, Bio-chemical characterization of a β-mannanase from Bacillus licheniformis HDYM-04 isolated from flax water-retting liquid and its decolorization ability of dyes, RSC Adv., 2016, 6, 23612–23621 RSC.
- M. G. I. Galinato, R. S. Fogle III, A. Stetz and J. F. Galan, Modulating the nitrite reductase activity of globins by varying the heme substituents: utilizing myoglobin as a model system, J. Inorg. Biochem., 2016, 154, 7–20 CrossRef CAS PubMed.
- G. Jin, L. He, X. Yu, J. Zhang and M. Ma, Antioxidant enzyme activities are affected by salt content and temperature and influence muscle lipid oxidation during dry-salted bacon processing, Food Chem., 2013, 141, 2751–2756 CrossRef CAS PubMed.
- W. G. Zumft, Cell biology and molecular basis of denitrification, Microbiol. Mol. Biol. Rev., 1997, 61, 533–616 CAS.
- R. Zhao, H. M. Zhang, Y. F. Li, T. Jiang and F. L. Yang, Research of iron reduction and the iron reductase localization of anammox bacteria, Curr. Microbiol., 2014, 69, 880–887 CrossRef CAS PubMed.
- Y. Wang, Z. Zhang, L. Qiu, Y. Guo, X. Wang, X. Xiong and S. Che, Effect of temperature downshifts on biological nitrogen removal and community structure of a lab-scale aerobic denitrification process, Biochem. Eng. J., 2015, 101, 200–208 CrossRef CAS.
- K. Nootong and W. K. Shieh, Analysis of an upflow bioreactor system for nitrogen removal via autotrophic nitrification and denitrification, Bioresour. Technol., 2008, 99, 6292–6298 CrossRef CAS PubMed.
- J. T. Duane, Learning Curve Approach to Reliability Monitoring, IEEE Trans. Aerosp., 1964, 21, 563–566 CrossRef.
- W. Nelson, Analysis of performance degradation data from accelerated tests, IEEE Trans. Reliab., 1981, 30, 149–155 CrossRef.
- J. L. Nielsen, S. Juretschko and M. Wagner, Abundance and phylogenetic affiliation of iron reducers in activated sludge as assessed by fluorescence in situ hybridization and microautoradiography, Appl. Environ. Microbiol., 2002, 68, 4629–4636 CrossRef CAS PubMed.
- R. V. Chopedkar, G. Hu, A. C. Ford and Y. Suzuki, Magnetics and Magnetoresistance in Epitaxial Magnetite Heterostructures, J. Electron. Mater., 2004, 33, 11 CrossRef.
- W. Chua-anusorn and J. Webb, Infrared spectroscopic studies of nanoscale iron oxide deposits isolated from human thalassemic tissues, J. Inorg. Biochem., 2000, 79, 303–309 CrossRef CAS PubMed.
- B. Pal, R. Kaur and I. S. Grover, Superior adsorption and photodegradation of eriochrome black-T dye by Fe3+ and Pt4+ impregnated TiO2 nanostructures of different shapes, J. Ind. Eng. Chem., 2016, 33, 178–184 CrossRef CAS.
- S. Teng and B. Li, Study of adsorption of organic pollutants by Attapulgite/Gamma-Fe2O3 carbon Nanocomposite, Guangzhou Chem. Ind., 2013, 41, 112–145 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 

![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) was the average concentration of microorganisms (mg L−1). C(t) was the concentration of nitrate (mg L−1) which was measured at time t (h). Moreover, in order to evaluate the reliability of the data at different temperatures, Arrhenius equation:20
was the average concentration of microorganisms (mg L−1). C(t) was the concentration of nitrate (mg L−1) which was measured at time t (h). Moreover, in order to evaluate the reliability of the data at different temperatures, Arrhenius equation:20
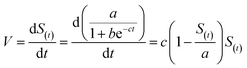



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/c. The data were presented in Table 1 through curves fitting by using the software Curve Expert 1.3. Meanwhile, the degradation rate of nitrate could be obtained and from the following deduction:
b/c. The data were presented in Table 1 through curves fitting by using the software Curve Expert 1.3. Meanwhile, the degradation rate of nitrate could be obtained and from the following deduction:
![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) was the average concentration of nitrate between t and t − 1 (t ≥ 1), K was the saturation constant when V = 0.5Vmax. The formula was regarded as the linear equation of y = nx + m, V−1 = y, x =
was the average concentration of nitrate between t and t − 1 (t ≥ 1), K was the saturation constant when V = 0.5Vmax. The formula was regarded as the linear equation of y = nx + m, V−1 = y, x = ![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) −1, K/Vmax = n, Vmax−1 = m, therefore, Vmax = 1/m and K = n/m could be obtained by linear fitting, m was the intercept of y-axis and n was the slope. Moreover, the kinetics parameters of nitrate removal were presented in Table 2. The Adj. R-square showed that the data more closer to 1, the better the correlation. And Pearson's r was also used as a test of significance for the data, when the Pearson's r was close to 1, showed that the data was relatively significant.21 Therefore, it could be indicated that the data were in agreement with the selected kinetic equation well as the r or R were close to 1 in this study.
−1, K/Vmax = n, Vmax−1 = m, therefore, Vmax = 1/m and K = n/m could be obtained by linear fitting, m was the intercept of y-axis and n was the slope. Moreover, the kinetics parameters of nitrate removal were presented in Table 2. The Adj. R-square showed that the data more closer to 1, the better the correlation. And Pearson's r was also used as a test of significance for the data, when the Pearson's r was close to 1, showed that the data was relatively significant.21 Therefore, it could be indicated that the data were in agreement with the selected kinetic equation well as the r or R were close to 1 in this study.