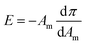Langmuir monolayer assisted formation of cadmium sulfide nanoparticles at the air–water interface and their role in the alignment of bulk liquid crystals
C. Karthik,
V. Manjuladevi and
R. K. Gupta *
*
Department of Physics, Birla Institute of Technology and Science, Pilani, Rajasthan – 333031, India. E-mail: raj@pilani.bits-pilani.ac.in; Fax: +91 1596 244183; Tel: +91 9828041535
First published on 21st April 2016
Abstract
Metal-sulfide nanoparticles can be fabricated by a one-step synthetic route by forming a Langmuir monolayer of long chain fatty acids over a subphase possessing metal ions and H2S gas in an air medium. Due to an interfacial reaction, the metal-sulfide nanoparticles are fabricated. The one-step synthesis can be further simplified by the appropriate choice of the amphiphilic molecules. The Langmuir monolayer of octadecanethiol (ODT) was reported to be stable over an ultrapure ion-free water subphase and can be perturbed significantly due to the presence of metal ions in the subphase. In this paper, we report the Langmuir monolayer assisted formation of CdS nanoparticles due to the interfacial interaction between the organosulfur compound, ODT and cadmium ions in the aqueous subphase. The films of nanoparticles were deposited on a hydrophilic quartz substrate by the Langmuir–Blodgett (LB) technique. UV-VIS spectra of the LB films of CdS nanoparticles at different target surface pressures and different Cd2+ ion concentrations depicted the signature of CdS nanoparticles. X-ray diffraction (XRD) measurements confirmed the crystalline structure of the CdS nanoparticles to be cubic. The average size of the CdS nanoparticles was estimated using field emission scanning electron microscopy, atomic force microscopy and XRD results and it was found to be in the range of 22–25 nm. We also incorporated CdS nanoparticles in the monolayer matrix of rod shaped liquid crystal (4-n-octyl-cyanobiphenyl, 8CB) molecules at an A/W interface, and transferred the film to solid substrates by the LB technique. Such LB films were employed as the alignment layer during the fabrication of liquid crystal cells and the alignment of bulk liquid crystal molecules in a nematic phase was studied using polarizing optical microscopy. The presence of CdS nanoparticles in the 8CB monolayer matrix reduces the surface anchoring of the liquid crystal molecule and thereby enhances the planar alignment of the bulk liquid crystal molecules.
Introduction
A Langmuir monolayer (LM) is a stable single layer of amphiphilic molecules adsorbed at the air–water interface (A/W). It is one of the widely employed systems used to study the thermodynamics of a two dimensional (2D) system wherein the 2D plane is provided by the smooth water surface.1,2 There are numerous reports on the studies of the LM of varieties of materials including fatty acids, cholesterol and derivatives, proteins, liquid crystals and nanomaterials. The LM systems are used to study the role of molecular interactions on the surface phases.3,4 The presence of ions in the aqueous subphase can strongly perturb the phases of the LM due to the formation of an organic/inorganic complex at the interface. Such complexation occurs at the A/W interface due to some surface chemistry.1,5 Taking advantage of the formation of metal ion–organic complexes at the A/W interface, there have been numerous attempts to fabricate nanoparticles assisted by the LM. Such nanoparticles can be transferred to solid substrates by the Langmuir–Blodgett (LB) or Langmuir–Schaefer (LS) method for device applications. The LB technique provides the flexibility to control the film thickness by depositing the material layer-by-layer at a given target surface pressure, and the surface density of nanoparticles to be deposited.3,6 The LB and LS methods were successfully used for the deposition of semiconducting nanoparticles onto different substrates to observe the structural morphology of the monolayer film, and its applications.7 The films of metal-sulfide nanoparticles including CdS, ZnS, and CuS were prepared and studied.8–12 In a one step synthetic procedure, these nanoparticles were fabricated by forming a LM of long chain fatty acids at the A/W interface and establishing an interfacial reaction between the metal ions in the subphase and H2S gas in an air medium in the presence of the monolayer of the fatty acid. This protocol is mostly adopted to synthesize the metal-sulfide nanoparticles at the A/W interface.13–17 The one step synthesis of metal-sulfide nanoparticles can be further simplified with an appropriate choice of the LM forming amphiphilic molecules. It has been reported that gold nanoribbons can be synthesized by forming a LM of hexadecylaniline on the aqueous subphase containing gold ions.18 The hexadecylaniline interacts with gold ions electrostatically, reduces them to gold nanoparticles and encapsulates the nanoparticles thus formed.19In this paper, we have formed a LM of octadecanethiol (ODT) molecules with cadmium ions in the aqueous subphase. The ODT molecules in the LM interact electrostatically with the cadmium ions of the subphase, form a complex and reduce it to form CdS nanoparticles at the A/W interface. The fabricated nanoparticles were characterized using UV-vis spectroscopy, atomic force microscopy, field emission scanning electron microscopy, X-ray diffraction and zeta potential measurements. CdS nanoparticles can be synthesized by various other techniques employing microwave irradiation, double-hydrophilic block copolymers, Fusarium oxysporum and a reverse micellar system.20–23 The CdS nanoparticles have been synthesized due to the chemical interaction between Cd2+ and S2− ions in the aqueous medium of a mixture of CdSO4 and (NH4)2S.24,25 In this article, our proposed synthetic route for the fabrication of CdS nanoparticles assisted by the LM of ODT is much simpler, faster and more economical.
Biological systems can be imaged by embedding the CdS nanoparticles into them.26,27 This can find application in microelectronics and optoelectrochemistry.28–30 In the present work, we have employed the CdS nanoparticles for a non-traditional application. The CdS nanoparticles were embedded in situ in the monolayer matrix of rod shaped liquid crystal molecules, 4-n-octyl-cyanobiphenyl (8CB). We employed the CdS incorporated LB films of 8CB as an alignment layer without rubbing and studied its role in the alignment of bulk liquid crystal (LC) molecules. We found that the presence of CdS nanoparticles in the 8CB matrix in the alignment layer enhances the technologically relevant planar alignment of the liquid crystal molecules. There are few reports on the application of LB films for aligning bulk liquid crystals.31–35
Experimental methods
The Langmuir monolayer of ODT was formed at the A/W interface with Cd2+ ions in the subphase. Due to the interfacial interaction between the ODT molecules and Cd2+ ions, the CdS nanoparticles form at the A/W interface. The LB film of CdS nanoparticles was transferred onto solid substrates for characterization. The CdS nanoparticles were embedded into the monolayer matrix of 8CB by forming a mixed monolayer of 8CB and ODT over the aqueous subphase containing Cd2+ ions. The LB films of the mixed monolayer of 8CB + ODT with Cd2+ in the subphase were transferred onto a glass substrate and these were employed as an alignment layer without rubbing for aligning bulk liquid crystals in a nematic phase.Materials
The materials, ODT and cadmium chloride were obtained from Sigma Aldrich. The organic solvents e.g. chloroform and alcohol were procured from Merck. The ultrapure ion free water having a resistivity ≥18 MΩ cm was obtained by circulating reverse osmosis water in the Millipore Milli-Q (DQ5) filtering system. The pH of the ion free water was 6.8. One-sided polished silicon (Si) wafers were obtained from Optochem Int. All experiments were done at 20 ± 0.3 °C.Methods
Results and discussion
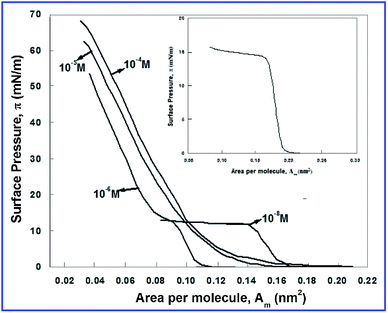
The π–Am isotherm of the ODT molecules at the interface of air and ultrapure ion free water (inset of Fig. 1) indicates that the monolayer exhibits a gas phase and a liquid condensed phase and a collapse. The collapse surface pressure was found to be around 14 mN m−1. The ODT molecules were found to form a stable monolayer at the A/W interface. The monolayer of ODT molecules was also found to be very sensitive to the addition of monovalent, divalent and trivalent ions in the aqueous subphase.36Fig. 1 shows the π–Am isotherms of ODT molecules on the aqueous subphase possessing 10−4, 10−5, 10−6 and 10−8 M concentrations of CdCl2 in ultrapure water. The isotherms show that even a very minute addition of Cd2+ ions into the water subphase results in significant deviation in the isotherm as compared to the monolayer of the ODT molecules on ion free water. As the concentration of CdCl2 in the subphase increases, there is a increase in the condensation of the ODT monolayer, and the isotherms shift towards lower values of Am. At higher concentrations of CdCl2 in the subphase, the surface pressure rises monotonically to be very high. The trend of isotherms for the concentrations of Cd2+ ions in subphase lower than 10−5 M shows the signature of the isotherm corresponding to that on ultrapure water. This indicates the partial complexation of the ODT monolayer with Cd2+ ions. The trend of the isotherm for concentrations ≥10−5 M changes completely which indicates complete complexation of the ODT with Cd2+ ions above such concentrations.
The isothermal in-plane elastic modulus (E) is an appropriate parameter to estimate the elastic nature of the phases of a Langmuir monolayer and for distinguishing very weak phase transition.37 The in-plane elastic modulus can be estimated from the isotherm using the relation
Fig. 2 shows the variation of E as a function of Am of ODT molecules for different concentrations of CdCl2 in the subphase. The variation of E for the ODT monolayer on the ion-free water subphase shows a peak corresponding to the untilted condensed phase. The maximum value of E in the condensed phase is around 120 mN m−1. As the concentration of the CdCl2 increases, the peak corresponding to the condensed phase shifts towards lower Am values. For the concentrations >10−6 M of CdCl2, the peak becomes broader and appears at lower Am values. This is due to the complete complexation of the ODT monolayer with the Cd2+ ions of the subphase.
 | ||
| Fig. 2 Plot of elastic modulus (E) vs. area per molecule (Am) of the octadecanethiol (ODT) monolayer with different concentrations of CdCl2 in the subphase. | ||
The LB films were deposited from the aqueous subphases of 10−4, 10−5 and 10−6 M of CdCl2 solution and at four different πt i.e. 5, 20, 30 and 40 mN m−1. The average transfer ratio was found to be 1 ± 0.12 which indicates the better physical adhesion of the ODT–Cd2+ complex onto the hydrophilic quartz plates. The LB films were scanned using an UV-VIS spectrophotometer and the spectra were collected.
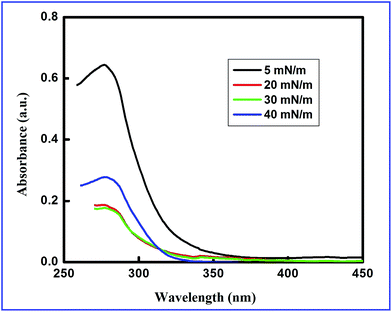
Fig. 3 shows UV-VIS spectra of the LB films of the ODT–Cd2+ complex deposited from the subphases with different concentrations of CdCl2 onto quartz plates at different πt. The energy gap (Eg) is estimated from the wavelength (λ) corresponding to the initial absorption.38 The UV-VIS spectra show λ at around 315 nm which is the signature of CdS nanoparticles.39 Table 1 shows the λ and corresponding Eg for the LB films of the ODT–Cd2+ complex deposited at different πt for different concentrations of CdCl2 in the aqueous subphase.
 | ||
| Fig. 3 UV-VIS spectra of the LB films of the ODT–Cd2+ complex deposited from the subphases with different concentrations of CdCl2 onto quartz plates at different target surface pressures (πt). | ||
| CdCl2 concentration in subphase | Target surface pressure (πt) (mN m−1) | Wavelength (λ) in nm | Energy gap (Eg) in eV |
|---|---|---|---|
| 10−4 M | 5 | 313 | 3.95 |
| 20 | 312 | 3.97 | |
| 30 | 311 | 3.99 | |
| 40 | 310 | 4.01 | |
| 10−5 M | 5 | 317 | 3.90 |
| 20 | 315 | 3.93 | |
| 30 | 313 | 3.95 | |
| 40 | 311 | 3.98 | |
| 10−6 M | 20 | 315 | 3.88 |
| 30 | 313 | 3.90 | |
| 40 | 311 | 3.95 |
The estimated values of Eg obtained from the LB films of the ODT–Cd2+ complex are close to the Eg value of cubic CdS nanoparticles i.e. 3.9 eV. This indicates that the ODT–Cd2+ complex formed at the A/W interface can be CdS nanoparticles with a crystalline cubic structure.40 The average Eg of the CdS nanoparticles was found to be increasing marginally with increasing concentration of Cd2+ ions in the aqueous subphase. This is shown in Fig. 4.
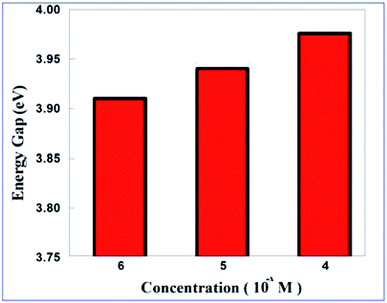 | ||
| Fig. 4 Plot of the average energy gap (Eg) of the LB films of the ODT–Cd2+ complex deposited from different concentrations (10−x M) of CdCl2 in the aqueous subphase. | ||
The LB films of the ODT–Cd2+ complex at different πt for the 10−5 M concentration of CdCl2 in the aqueous subphase were ultrasonicated in chloroform solvent for about 30 minutes. The nanoparticles desorbed from the substrate and their suspension in chloroform was scanned using an UV-VIS spectrometer. The spectra are shown in Fig. 5. The results are summarized in Table 2. The Eg values decreased by a very small amount compared to the Eg values of the LB films of the ODT–Cd2+ complex deposited onto the quartz substrate. The decrease in Eg values is due to the solvent effect.41
| CdCl2 concentration in subphase | Target surface pressure (πt) (mN m−1) | Wavelength (λ, nm) | Energy gap Eg, (eV) |
|---|---|---|---|
| 10−5 M | 5 | 355 | 3.48 |
| 20 | 350 | 3.53 | |
| 30 | 344 | 3.59 | |
| 40 | 328 | 3.77 |
The spectroscopic study reveals the formation of CdS nanoparticles. In earlier reports, it was found that gold nanoparticles can be synthesized in a simplified single step involving the interfacial interaction between the hexadecylaniline and gold ions.18,19 In the present case, the Cd2+ ions in the aqueous medium interact with the –SH group of the ODT molecules at the A/W interface and such interfacial interaction may lead to the formation of CdS nanoparticles.
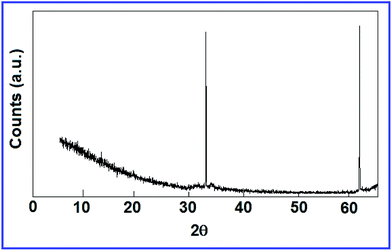
The crystalline structure of the CdS nanoparticles was studied using the results of X-ray diffraction measurements (Fig. 6). Prominent peaks were observed at 2θ = 32.5° and 61° which indicate the crystalline structure of the CdS nanoparticles to be cubic.42,43 Using the Scherrer equation,44 we found the size of the nanoclusters to be 25 nm.
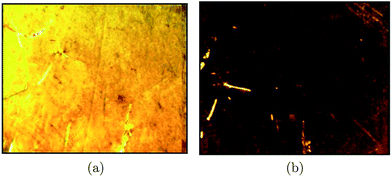
The surface morphology of the LB films of the ODT–Cd2+ complex deposited at 30 mN m−1 onto the Si substrate at the 10−4 M concentration of CdCl2 was studied through FESEM (Fig. 7) and AFM (Fig. 8). The FESEM image (Fig. 7a) shows small white particles distributed over a gray background. These bright particles are the CdS nanoparticles. These particles also aggregate to form large clusters on the Si substrate. The scanning areas were zoomed and focused on the less dense region to image well separated CdS nanoparticles on the substrate. This is shown in Fig. 7b. The average size of the particles is estimated and found to be 24 ± 4 nm. Fig. 8 shows the AFM images of the LB film of the ODT–Cd2+ complex deposited at 30 mN m−1. The small spherical features in the images are due to the CdS nanoparticles. The size of the particles was estimated from the AFM image using granulometry and it was found to be 22 ± 3 nm.
A dispersion of CdS nanoparticles in ethanol solvent was obtained by ultrasonicating the LB films of the ODT–Cd2+ complex deposited at 30 mN m−1 from the aqueous subphase of the 10−4 M concentration of CdCl2. In order to understand the stability of the nanoparticles against coagulation, zeta potential measurements of the nanoparticle suspension were performed and the results are shown in Fig. 9. The curve peaked at a negative zeta potential value i.e. −11.2 mV. This value indicates a partial stability of CdS nanoparticles in the ethanol solvent. The magnitude of the zeta potential gives an indication of the stability of the colloidal system. If the particles in the suspension have a large negative or positive zeta potential, then they will tend to repel each other and thereby the stability against coagulation increases. However, if the particles have a low magnitude of zeta potential values then due to weak interparticle repulsive forces, they tend to coagulate.45,46 The magnitude of the zeta potential obtained from the ethanol suspension of CdS nanoparticles shifted towards lower values with time when the measurement was performed at an interval of 24 hours. This indicates that the CdS nanoparticles in ethanol solvent aggregate to form clusters with increasing time.
 | ||
| Fig. 9 Zeta potential results of the ethanol dispersed LB film of the ODT–Cd2+ complex deposited at 30 mN m−1 from the aqueous subphase of the 10−4 M concentration of CdCl2. | ||
The LB film consisting of CdS nanoparticles can be transferred to the active area of a device for technological applications. With the proposed methodology, the CdS nanoparticles can also be grown at random local sites in the monolayer matrix of another material. The incorporation of nanoparticles can also provide an avenue for altering the properties of the LB film by altering the density of the nanoparticles. The alignment of a bulk liquid crystal (LC) in a constrained geometry is one of the key issues for display applications. There have been few attempts to align LCs in a nematic phase using LB films of different materials without mechanical rubbing.31–35 However, most reported LC alignment using LB films is homeotropic. The alignment of bulk LC primarily depends on the morphology of the alignment layer. The planar or homogeneous alignment of bulk LC can be achieved by altering the morphology of the alignment layer on the solid substrate.47
In the present work, we embedded the CdS nanoparticles in situ in the LM of rod-shaped amphiphilic liquid crystal molecules, and transferred the LB films of such nanocomposites to solid substrates. Such LB films were employed as an alignment layer during the fabrication of the LC-cells and the alignment of bulk LC molecules was studied using such LC-cells. We formed the LM of the mixed components of 8CB and ODT over the aqueous subphase possessing Cd2+ ions and carried out systematic surface manometry studies on such a mixed system. The π–Am isotherms of the monolayers of pure 8CB and 8CB mixed with different mole percents of ODT on an ultrapure ion-free water subphase is shown in Fig. 10a. The isotherm shifts towards lower Am values with increasing concentration of ODT in 8CB and with the incorporation of Cd2+ ions in the subphase (Fig. 10b). This indicates condensation of the LM of 8CB due to such incorporation. The monolayer condenses as the amount of ODT molecules in the 8CB matrix increases on the ion free water. The condensation of the monolayer over a 10−4 M concentration of CdCl2 in the aqueous subphase is further enhanced due to the formation of CdS nanoparticles by involving a larger number of ODT molecules. The trend of the isotherms of the mixed monolayers of 8CB + ODT is similar to that of pure 8CB. This indicates that the surface phase of the 8CB is not perturbed largely due to incorporation of ODT and ODT with Cd2+ ions in the subphase.
The AFM image of the LB film of 8CB with 10 mole percent of ODT deposited at πt = 2 mN m−1 from the ultrapure ion-free water subphase is shown in Fig. 11a. The image appears homogeneous over the scan area of 4 × 4 μm2. This indicates that ODT and 8CB may mix well at this proportion in the LB film. Interestingly, the AFM image of LB film of 8CB with 10 mole percent of ODT deposited at the target surface pressure of 2 mN m−1 from the aqueous subphase containing the 10−4 M concentration of CdCl2 (Fig. 11b) shows bright dots on the uniform background. The bright dots represent clusters of CdS nanoparticles embedded uniformly in the matrix of the 8CB monolayer.
The LB films of 8CB with 10 mole percent of ODT were deposited onto glass slides at πt = 2 mN m−1 from the ultrapure ion-free water subphase and aqueous subphase containing the 10−4 M concentration of CdCl2. The glass slides deposited with the LB films were used for the fabrication of the LC-cells. A nematic LC, E7, was used to fill the cell. The LC-cells were placed under a polarizing microscope (Olympus BX51) and the textures were observed in the transmission mode. Fig. 12 shows the texture of the LC-cell fabricated using the LB film of 8CB with 10 mole percent of ODT deposited from ultrapure ion free water as the alignment layer. On rotating the stage fixed with the LC-cell between the crossed polarizers of the microscope, the intensity dropped from maximum to minimum but did not get extinguished. This indicates a tendency of planar alignment of the nematic LC, E7. Fig. 13 shows the texture of the LC-cell fabricated using the LB film of 8CB with embedded CdS nanoparticles. Here, on rotation of the stage fixed with the LC-cell between the crossed polarizers of the microscope, we observed a drop in intensity from maximum to zero. This indicated planar alignment of the E7 LC.
The role of the interaction of the aliphatic chain of the LB films of fatty acids on the alignment of bulk LC has been studied. With the increase in separation from the substrate by an increase in the length of the aliphatic chain, the anchoring strength reduces and the LC molecules prefer a planar orientation.48 The bumps of BiFeO3 in the alignment layer facilitate the planar alignment of the LC molecules.47
The LB film of 8CB + ODT molecules offers a uniform layer with the aliphatic chain towards the air medium. Such morphology supports the tendency of planar orientation of bulk LCs. The presence of clusters of CdS nanoparticles along with the 8CB molecules in the alignment layer (LB film) causes the LC molecules to exhibit a pre-tilt in the vicinity of the cluster of the nanoparticles. Such a pre-tilt in the interfacial region of the alignment layer and LC medium facilitates the planar orientation of LC molecules. Hence the presence of CdS nanoparticles in the 8CB matrix in the alignment layer favours the planar alignment of the bulk LCs. Such a technique can be employed in LC display industries.
Conclusions
The Langmuir monolayer can be employed for the formation of nanomaterials at the A/W interface. Such nanomaterials can be transferred onto an active area for technological application. In the present work, we demonstrated a simpler one step synthesis of CdS nanoparticles assisted by the Langmuir monolayer (LM) of ODT on the aqueous subphase of CdCl2. Such nanoparticles can be embedded in situ in the monolayer matrix of some other material. The CdS nanoparticles were embedded in the LM of a rod shaped liquid crystal, 8CB. The LB films of 8CB + CdS nanoparticles were deposited and employed as an alignment layer for the fabrication of LC-cells. The alignment of the bulk LC, E7, was studied under such geometry. The presence of the CdS nanoparticles favours the planar alignment of E7 in the LC-cell.Acknowledgements
The authors (BITS, Pilani) are thankful to University Grants Commission of India for their support under the UGC SAP programme. We thank UGC for the BSR fellowship. We thank Prof Sandeep Kumar of Raman Research Institute for useful discussion. We are also thankful to Mrs K. N. Vasudha for helping us in conducting XRD experiments. Thanks are also due to Mr A. Dhason for assisting in FESEM.References
- G. L. Gaines, Insoluble Monolayers at Liquid-Gas Interfaces, Wiley-Interscience, New York, 1966 Search PubMed
.
- R. K. Gupta, and V. Manjuladevi Molecular Interaction, Intech, Croatia, 2012, ch. 4 Search PubMed
.
- G. Roberts, Langmuir–Blodgett Films, Plenum, New York, 1990 Search PubMed
.
- V. M. Kaganer, H. Möhwald and P. Dutta, Rev. Mod. Phys., 1999, 71, 779 CrossRef CAS
.
- A. Junghans, C. Champagne, P. Cayot, C. Loupiac and I. Coper, Langmuir, 2010, 26, 12049 CrossRef CAS PubMed
.
- R. K. Gupta, and V. Manjuladevi, Comprehensive Guide for Nanocoatings Technology, Nova Publishers, New York, 2015, ch. 10 Search PubMed
.
- N. F. Crawford and R. M. Leblanc, Coord. Chem. Rev., 2014, 263–264, 13 CrossRef CAS
.
- Y. Yuan, I. Cabasso and J. H. Fendler, Chem. Mater., 1990, 2, 226 CrossRef CAS
.
- K. C. Yi and J. H. Fendler, Langmuir, 1990, 6, 1519 CrossRef CAS
.
- X. K. Zhao, L. D. McCormick and J. H. Fendler, Chem. Mater., 1991, 3, 922 CrossRef CAS
.
- X. K. Zhao and J. H. Fendler, J. Phys. Chem., 1991, 95, 3716 CrossRef CAS
.
- X. K. Zhao, S. Xu and J. H. Fendler, Langmuir, 1991, 7, 520 CrossRef CAS
.
- W. L. Yu, W. Huang, B. Y. Zhu and G. X. Zhao, Mater. Lett., 1997, 33, 221 CrossRef CAS
.
- Z. Y. Pan, X. J. Liu, S. Y. Zhang, L. G. Zhang, Z. H. Lu and J. Z. Liu, J. Phys. Chem. B, 1997, 101, 9703 CrossRef CAS
.
- C.-W. Wang, H.-G. Liu, X.-T. Bai, Q. Xue, X. Chen, Y.-I. Lee, J. Hao and J. Jiang, Cryst. Growth Des., 2008, 8, 2660 CAS
.
- G.-Q. Xin, H.-P. Ding, Y.-G. Yang, S.-L. Shen, Z.-C. Xiong, X. Chen, J. Hao and H.-G. Liu, Cryst. Growth Des., 2009, 9, 2008 CAS
.
- Y.-G. Yang, H.-G. Liu, L.-J. Chen, K.-C. Chen, H.-P. Ding and J. Hao, Langmuir, 2010, 26, 14879 CrossRef CAS PubMed
.
- A. Swami, A. Kumar, P. R. Selvakannan, S. Mandal, R. Pasricha and M. Sastry, Chem. Mater., 2003, 15, 17 CrossRef CAS
.
- P. R. Selvakannan, S. Mandal, R. Pasricha, S. D. Adyanthaya and M. Sastry, Chem. Commun., 2002, 1334–1335 RSC
.
- L. Qi, H. Colfen and M. Antoniett, Nano Lett., 2001, 1, 61 CrossRef CAS
.
- A. Ahmad, P. Mukherjee, D. Mandal, S. Senapati, M. I. Khan, R. Kumar and M. Sastry, J. Am. Chem. Soc., 2002, 124, 12108 CrossRef CAS PubMed
.
- J. Zhu, M. Zhou, J. Xu and X. Liao, Mater. Lett., 2001, 47, 25 CrossRef CAS
.
- A. Agostiano, M. Catalano, M. Curri, M. Della Monica, L. Manna and L. Vasanelli, Micron, 2000, 31, 253 CrossRef CAS PubMed
.
- T. Trindade, P. O’Brien and N. L. Pickett, Chem. Mater., 2001, 13, 3843 CrossRef CAS
.
- R. Rossetti, J. L. Ellison, J. M. Gibson and L. E. Brus, J. Chem. Phys., 1984, 80, 4464 CrossRef CAS
.
- M. Bruchez, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013 CrossRef CAS PubMed
.
- W. C. W. Chan and S. M. Nie, Science, 1998, 281, 2016 CrossRef CAS PubMed
.
- S. Chaudhary, M. Ozkan and W. C. W. Chan, Appl. Phys. Lett., 2004, 84, 2925 CrossRef CAS
.
- V. Colin, M. C. Schlamp and A. P. Alivisatos, Nature, 1994, 370, 374 Search PubMed
.
- C. J. Murphy and J. L. Coffer, Appl. Spectrosc., 2002, 56, 16A CrossRef CAS
.
- T. Sasaki, H. Fujii and M. Nishikawa, Jpn. J. Appl. Phys., 1992, 31, L632 CrossRef CAS
.
- A. Modlinska, J. Makowiecki, D. Bauman and T. Martynski, Liq. Cryst., 2013, 40, 831 CrossRef CAS
.
- V. V. Belyaev, V. G. Mazaeva, M. V. Sobolevskii, I. G. Kokaulina and A. S. Solomatin, Mol. Cryst. Liq. Cryst., 2011, 546, 17 CAS
.
- A. Modlinska, K. Inglot, T. Martynski, R. Dabrowski, J. Jadzyn and D. Bauman, Liq. Cryst., 2009, 36, 197 CrossRef CAS
.
- T. Michinobu, K. Nakada, M. Matsuda and K. Shigehara, Colloids Surf., A, 2008, 321, 82 CrossRef CAS
.
- R. K. Gupta, K. Suresh, R. Guo and S. Kumar, Anal. Chim. Acta, 2006, 568, 109 CrossRef CAS PubMed
.
- H. Möhwald, Handbook of Biological Physics, Elsevier Science, Amsterdam, 1995, ch. 4 Search PubMed
.
- A. S. Bhadwal, R. M. Tripathi, R. K. Gupta, N. Kumar, R. P. Singh and A. Shrivastav, RSC Adv., 2014, 4, 9484 RSC
.
- S. L. Murov, G. L. Hug, and I. Carmichael, Handbook of Photochemistry, CRC Press, US, 1993 Search PubMed
.
- R. Banerjee, R. Jayakrishnan and P. Ayyub, J. Phys.: Condens. Matter, 2000, 12, 10647 CrossRef CAS
.
- G. Ramakrishna and H. N. Ghosh, Langmuir, 2003, 19, 505 CrossRef CAS
.
- B. E. Warren, X-ray Diffraction, Dover Publications, 1969 Search PubMed
.
- M. Protiére, N. Nerambourg, O. Renard and P. Reiss, Nanoscale Res. Lett., 2011, 6, 472 CrossRef PubMed
.
- A. Patterson, Phys. rev., 1939, 56, 978 CrossRef CAS
.
- G. Greenwood and K. Kendall, J. Eur. Ceram. Soc., 1999, 19, 479 CrossRef
.
- D. A. H. Hanaor, M. Michelazzi, C. Leonelli and C. C. Sorrell, J. Euro. Cer. Soc., 2012, 32, 235 CrossRef CAS
.
- Y. Liu, K. Liu, P. Wen, B. Y. Oh, H. G. Park and D. S. Seo, Liq. Cryst., 2016, 43, 320 CrossRef CAS
.
- A. L. Alexe-lonescu, G. Barbero and L. Komitov, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 80, 021701 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |