DOI:
10.1039/C6RA08117G
(Paper)
RSC Adv., 2016,
6, 52957-52965
Facile morphology controlled synthesis of nanostructured Co3O4 films on nickel foam and their pseudocapacitive performance†
Received
29th March 2016
, Accepted 23rd May 2016
First published on 26th May 2016
Abstract
Nanostructured transition metal oxides are a current investigation focus for supercapacitors. We herein report a facile solvothermal synthesis of nanostructured Co3O4 films on nickel foam. The morphologies and dimensions of the Co3O4 films can be effectively tuned by tailoring the solvent compositions in the solvothermal reaction solutions. The effect of solvent composition on the morphologies of nanostructured Co3O4 films is analyzed. The 3D hierarchically porous Co3O4 network film, which is synthesized in the solvothermal reaction solution with an intermediate ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29), shows a markedly enhanced pseudocapacitive performance. The specific capacitances of the Co3O4 network film electrode at the current densities of 0.870 and 17.391 A g−1 are 2817 and 1948 F g−1, respectively, revealing its large specific capacitance and excellent rate capability. Furthermore, the Co3O4 network film electrode exhibits good electrochemical cycling stability with a specific capacitance of 1628 F g−1 after 3500 cycles at a current density of 4.348 A g−1. The prominent pseudocapacitive performance of the Co3O4 network film electrode can be attributed to its unique structural characteristics. The as-synthesized 3D hierarchically porous Co3O4 network film with excellent pseudocapacitive performance demonstrates promising potential as a high-performance electrode for supercapacitors.
29), shows a markedly enhanced pseudocapacitive performance. The specific capacitances of the Co3O4 network film electrode at the current densities of 0.870 and 17.391 A g−1 are 2817 and 1948 F g−1, respectively, revealing its large specific capacitance and excellent rate capability. Furthermore, the Co3O4 network film electrode exhibits good electrochemical cycling stability with a specific capacitance of 1628 F g−1 after 3500 cycles at a current density of 4.348 A g−1. The prominent pseudocapacitive performance of the Co3O4 network film electrode can be attributed to its unique structural characteristics. The as-synthesized 3D hierarchically porous Co3O4 network film with excellent pseudocapacitive performance demonstrates promising potential as a high-performance electrode for supercapacitors.
Introduction
The ever-increasing energy needs and the impending concerns of environmental problems have urgently called for the development of sustainable and highly efficient energy storage devices and systems. Among the promising electrical energy storage devices, electrochemical capacitors (supercapacitors) have become eye-catching due to their advantages of high power density, fast recharge ability and excellent cycling lifespan compared to batteries and conventional capacitors.1–4 According to the different charge storage mechanisms, electrochemical capacitors can be classified into two categories: electric double layer capacitors (EDLCs), with carbonaceous materials as the most commonly used materials,5,6 and pseudocapacitors, with transition metal oxides/hydroxides such as RuO2,7 MnO2,8,9 Co3O4,10–14 Ni(OH)2,15,16 or conducting polymers17 as active materials. The capacitance of EDLCs originates from the charge separation process at the electrode/electrolyte interface.3 In contrast, pseudocapacitors demonstrate larger specific capacitance and higher energy density than EDLCs, in which charge is stored by reversible surface redox reactions.15 The transition metal oxides and hydroxides have been extensively investigated as promising electrode materials for pseudocapacitors.
Among the investigated electrode materials, Co3O4 is regarded as a promising candidate for supercapacitors owing to its high theoretical capacitance, good electrochemical capability, low environment footprint and improved safety.10,18 However, the low electrical conductivity and small specific surface area of Co3O4 result in its relatively small actual capacitance, poor rate capability and/or low electrochemical cycling stability. In order to improve the capacitance performance of Co3O4, various strategies have been probed to prepare diverse nanostructured materials with large surface area to shorten the diffusion paths of ions and electrons. Various Co3O4 nanostructures have been reported as electrode materials for supercapacitors, including nanosheets,19,20 nanotubes,21 nanowires,22–24 nanoparticles,25,26 and other nanostructures.27–29 It is demonstrated that the morphology and dimension of Co3O4 play an important role in its pseudocapacitance properties. Some methods have been developed to synthesize nanostructured Co3O4 materials with controlled morphologies, however, most of them use template and structure-directing agents.20,21,29 The removal of these foreign substances leads to the complexity of synthetic processes and the possible introduction of some impurities. Facile morphology controlled synthesis of the Co3O4 nanomaterials with prominent pseudocapacitance performance still remains a great challenge.
In this work, various nanostructured Co3O4 films on nickel foam substrate are synthesized by the facile solvothermal method using ethylene glycol/water mixed solvents. It is found that the morphologies and dimensions of the Co3O4 films can be conveniently tuned by tailoring the solvent compositions in the solvothermal reaction solutions. A possible effect mechanism of solvent composition on the morphologies of Co3O4 films is proposed. The as-synthesized 3D hierarchically porous Co3O4 network film electrode shows large specific capacitance, excellent rate capability and good electrochemical-cycling stability. The reasons that the 3D hierarchically porous Co3O4 network film electrode shows prominent pseudocapacitive performance are discussed in detail.
Experimental
Preparation of Co3O4 nanostructures on nickel foam
The Co3O4 nanostructures were prepared by a solvothermal synthesis and subsequent thermal treatment. The reaction solutions consisted of 5 mM Co(NO3)2·6H2O, 10 mM NH4F and 25 mM CO(NH2)2 in an ethylene glycol/water mixed solvent with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 1
9, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and 1
29 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59. Prior to the synthesis, Ni foam (10 mm × 45 mm in rectangular shape) was treated in 2 M HCl solution for 10 min to remove the NiO surface layer, and ultrasonically cleaned in deionized water and acetone for 10 min, respectively. The reaction solution was vigorously stirred at room temperature. The as-prepared pink-colored homogeneous solution was then transferred into a 45 mL Teflon-lined stainless steel autoclave. After that, a piece of Ni foam with an exposed area of 10 × 20 mm2 (other section was protected from solution contamination by uniformly coating with a polytetrafluoroethylene tape) was then placed at an angle against the Teflon-lined wall of the stainless steel autoclave and immersed into the reaction solution. The sealed autoclave was maintained at 120 °C for 5 h in an electric oven. After synthesis, the autoclave was naturally cooled to room temperature. The substrate with the surface layer was rinsed with distilled water and ethanol, respectively. After dried at 60 °C for 10 h, the film sample was annealed at 250 °C in air for 3 h in a Muffle furnace. Careful weighing revealed that the loading masses of Co3O4 in various film samples were in the range of 0.9–1.16 mg cm−2. In the following discussions, the Co3O4 films synthesized in the ethylene glycol/water mixed solvents with volume ratios of 1
59. Prior to the synthesis, Ni foam (10 mm × 45 mm in rectangular shape) was treated in 2 M HCl solution for 10 min to remove the NiO surface layer, and ultrasonically cleaned in deionized water and acetone for 10 min, respectively. The reaction solution was vigorously stirred at room temperature. The as-prepared pink-colored homogeneous solution was then transferred into a 45 mL Teflon-lined stainless steel autoclave. After that, a piece of Ni foam with an exposed area of 10 × 20 mm2 (other section was protected from solution contamination by uniformly coating with a polytetrafluoroethylene tape) was then placed at an angle against the Teflon-lined wall of the stainless steel autoclave and immersed into the reaction solution. The sealed autoclave was maintained at 120 °C for 5 h in an electric oven. After synthesis, the autoclave was naturally cooled to room temperature. The substrate with the surface layer was rinsed with distilled water and ethanol, respectively. After dried at 60 °C for 10 h, the film sample was annealed at 250 °C in air for 3 h in a Muffle furnace. Careful weighing revealed that the loading masses of Co3O4 in various film samples were in the range of 0.9–1.16 mg cm−2. In the following discussions, the Co3O4 films synthesized in the ethylene glycol/water mixed solvents with volume ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 1
9, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and 1
29 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 were designated as WE1, WE9, WE29 and WE59, respectively.
59 were designated as WE1, WE9, WE29 and WE59, respectively.
Physical characterization
The surface morphologies and microstructures of the samples were characterized by scanning electron microscopy (SEM) using a ZEISS, ULTRA™ 55 with an accelerating voltage of 5 kV and transmission electron microscope (TEM HT7700) with an accelerating voltage of 120 kV. The structure was analyzed using a Rigaku D/Max 2550 X-ray diffractometer with Cu Kα radiation at 40 kV and 300 mA. Raman spectra were recorded using a Jobin Yvon Labor Raman series HR-800 with an excitation wavelength of 514 nm. The X-ray photoelectron spectroscopy (XPS) analysis was performed by a PHI 5000C X-ray physical electronics photoelectron spectrometer with Mg Kα radiation at 15 kV and 500 W. The binding energies were calibrated with respect to the adventitious C 1s peak, referenced at 284.6 eV. The specific surface area was measured by the Brunauer–Emmett–Teller (BET) method based on nitrogen adsorption/desorption using Micromeritics ASAP 2020 at 77 K. The specific surface area was calculated by BET method and the desorption isotherm was used to determine the pore size distribution via the Barrett–Joyner–Halenda (BJH) and Horvath–Kawazoe (HK) methods. The pore volume was obtained from the pore size distribution data. The masses of various films were determined by a microbalance (Sartorius BT25S).
Electrochemical tests
Electrochemical measurements were performed in a typical three-electrode glass cell with a platinum counter electrode and a Ag/AgCl reference electrode. Various film electrodes were used as the working electrode, and 6.0 M KOH solution was employed as electrolyte. Cyclic voltammetry (CV) was conducted at a scanning rate of 5 mV s−1 using a potentiostat (CHI 630D). Electrochemical impedance spectroscopy (EIS) measurements were carried out by an electrochemical analyzer (Parstat 2273), with the frequency range of 100 kHz to 0.01 Hz and a.c. signal amplitude of 10 mV. Galvanostatic charge/discharge (CD) tests were measured between the potential of 0–0.35 V by a potentiostat (Arbin BT-2000, USA).
The areal capacitance (CA), specific capacitance (C), energy density (de) and power density (dp) from the charge/discharge curves can be calculated in terms of eqn (1)–(4), respectively.15,20
| |
 | (1) |
| |
 | (2) |
| |
 | (3) |
| |
 | (4) |
where
S is the geometric area of the working electrode (cm
2),
m is the mass of active materials (g), Δ
V is the discharge potential window (V),
I is the discharge current (A), and Δ
t (s) is the discharge time.
In all the above experiments, the solutions were prepared from analytical reagents and deionized water.
Results and discussion
The XRD patterns of the as-synthesized WE9, WE29 and WE59 samples are displayed in Fig. 1a. The diffraction peaks at 2θ = 18.9°, 31.2°, 36.7°, 38.4°, 44.7°, 55.5°, 59.2°, 65.1° and 77.2° can be indexed as crystal planes (111), (220), (311), (222), (400), (422), (511), (440) and (533) for spinel Co3O4 phase in terms of JCPDS file 42-1467, respectively. Fig. 1b presents the Raman spectra of various films. Two main peaks at 516 and 680 cm−1 are attributable to the Co3+–O stretching vibrations in an octahedral site and the Co2+–O stretching vibrations in a tetrahedral site of Co3O4 lattice, respectively.21 The film (WE29) is further characterized by XPS, and the results are shown in Fig. 2. The wide-scan XPS spectrum in Fig. 2a indicates the existence of Co, Ni, C and O elements in the film. The Ni and C elements result from the nickel foam substrate and the adventitious carbon as the calibration reference, respectively. The two peaks at binding energies of 795.6 and 780.1 eV in the Co 2p XPS spectrum (Fig. 2b) can be attributed to the characteristic Co 2p1/2 and Co 2p3/2 spin–orbit peaks of Co3O4, respectively.30 The above results confirm the formation of the Co3O4 film on nickel foam.
 |
| | Fig. 1 XRD patterns (a) and Raman spectra (b) of various annealed films synthesized in the solvothermal reaction solutions with different volume ratios of ethylene glycol/water. The powder materials scratched from nickel foam were used for the XRD characterizations. | |
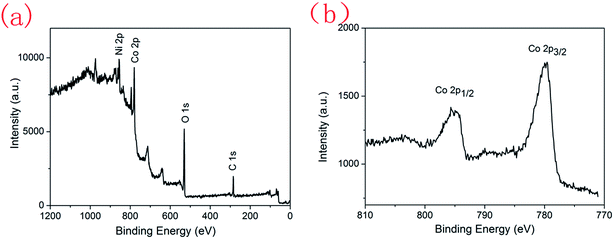 |
| | Fig. 2 Wide-scan XPS spectrum (a) and Co 2p XPS spectrum (b) of the annealed film (WE29) synthesized in the solvothermal reaction solution with an ethylene glycol/water volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29. 29. | |
The SEM images of the Co3O4 films synthesized on nickel foam in the reaction solutions with different ratios of ethylene glycol/water are illustrated in Fig. 3 and S1.† It is interesting to note that various nanostructured Co3O4 films are obtained in the reaction solutions with different solvent compositions. As illustrated in Fig. 3a, b and S1,† the solvothermal syntheses in the solutions with high volume ratios of ethylene glycol/water result in the growth of 2D Co3O4 nanosheets on nickel foam. These Co3O4 nanosheets lie aslant or perpendicular to the substrates, and are interconnected with each other, forming the nanosheet array structures. The thickness of the nanosheets significantly lowers as the ethylene glycol/water volume ratio decreases from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. A vertically aligned array of Co3O4 nanowires with a diameter of ∼100 nm is synthesized in the solution with a low ethylene glycol/water volume ratio (1
9. A vertically aligned array of Co3O4 nanowires with a diameter of ∼100 nm is synthesized in the solution with a low ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59), as shown in Fig. 3e and f. The Co3O4 nanowires separately stem from nickel foam substrate, and converge on various points of the top sections, leading to the formation of a bundle-like structure. For the Co3O4 film synthesized in the solution with a medium ethylene glycol/water volume ratio (1
59), as shown in Fig. 3e and f. The Co3O4 nanowires separately stem from nickel foam substrate, and converge on various points of the top sections, leading to the formation of a bundle-like structure. For the Co3O4 film synthesized in the solution with a medium ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29), as shown by the SEM images in Fig. 3c and d a unique Co3O4 network structure is observed, which is formed by the interconnected ultrathin nanosheets on the top surface.
29), as shown by the SEM images in Fig. 3c and d a unique Co3O4 network structure is observed, which is formed by the interconnected ultrathin nanosheets on the top surface.
 |
| | Fig. 3 SEM images of the Co3O4 films synthesized in the solvothermal reaction solutions with different volume ratios of ethylene glycol/water. (a, b) WE9, (c, d) WE29, (e, f) WE59. | |
The TEM and HRTEM images of the Co3O4 samples are demonstrated in Fig. 4, providing an insight of individual morphology and microstructure. The WE9 and WE59 samples are confirmed to have nanosheet (Fig. 4a) and nanowire (Fig. 4e) structures, respectively. While the WE29 sample exhibits a mixed structure of nanowires and ultrathin nanosheets (Fig. 4c). The HRTEM images in Fig. 4b, d and f reveal that all the three samples have a multiphase structure with lattice spacing values of 0.21, 0.24 and 0.29 nm, corresponding to those of the (400), (311) and (220) crystallographic planes of Co3O4. It is noted from Fig. 4 that these nanowires and nanosheets are composed of numerous nanoparticles with a size of several nanometers, exhibiting a mesopore structure. Combining the mesopore structure of the nanowires/nanosheets with the macropore feature of the array structure, the Co3O4 films on nickel foam demonstrate a 3D hierarchically porous architecture.
 |
| | Fig. 4 TEM images of the Co3O4 films synthesized in the solvothermal reaction solutions with different volume ratios of ethylene glycol/water. (a, b) WE9, (c, d) WE29, (e, f) WE59. | |
Further insight into the specific surface area and microstructure of the as-synthesized Co3O4 nanostructures are obtained by BET gas-sorption measurements, and the related results are shown in Fig. 5. Fig. 5a, c and e give the nitrogen adsorption/desorption isotherms of the three Co3O4 samples. The characteristic type IV isotherms with a type H3 hysteresis loop at the relative pressures of 0.5–0.8 indicate the highly mesoporous structures of the Co3O4 samples. The BET specific surface areas of the WE9, WE29 and WE59 samples are calculated to be 80.87, 83.13 and 72.76 m2 g−1, respectively, which are much larger than the corresponding values of typical mesoporous Co3O4 nanowires and porous Co3O4 nanosheets.12,31,32 Fig. 5b, d and f present the Barrett–Joyner–Halenda (BJH) pore-size distribution curves. The pore sizes of the three Co3O4 samples are mainly distributed in the range of 2–8 nm. This confirms the mesopore structure of the Co3O4 samples, resulting from the phase conversion and the release of gases during the thermal treatment of the precursor.
 |
| | Fig. 5 Nitrogen adsorption/desorption isotherms and pore-size distribution curves of the Co3O4 films synthesized in the solvothermal reaction solutions with different volume ratios of ethylene glycol/water. (a, b) WE9, (c, d) WE29, (e, f) WE59. | |
The chemical reactions involved in the synthesis process of Co3O4 can be expressed with the following formulas:33,34
| | |
Co2+ + xF− → CoFx(x−2)−
| (5) |
| | |
H2NCONH2 + H2O → 2NH3 + CO2
| (6) |
| | |
CO2 + H2O → CO32− + 2H+
| (7) |
| | |
NH3 + H2O → NH4+ + OH−
| (8) |
| | |
CoFx(x−2)− + 0.5CO32− + OH− + 0.11H2O → Co(CO3)0.5(OH)·0.11H2O + xF−
| (9) |
| | |
3Co(CO3)0.5(OH)·0.11H2O + 0.5O2 → Co3O4 + 1.83H2O + 1.5CO2
| (10) |
Co(CO3)0.5(OH)·0.11H2O precipitate is prepared by reactions 5–9 during the solvothermal synthesis, as confirmed by the XRD pattern in Fig. S2.† The final product Co3O4 is obtained by the thermal treatment of the precipitate (reaction (10)). The morphologies of the Co3O4 films are predominately determined by those of the precipitate precursor films.33,34 Most interestingly, it is noted in our work that the morphologies and dimensions of Co3O4 films can be effectively tuned by only altering the solvent compositions in the solvothermal reaction solutions. The dependences of the morphologies of the precursor films on the solvent compositions of the reaction solutions are schematically illustrated in Fig. 6. Generally, the formation of precipitate precursors on nickel foam involves initial nucleation and subsequent growth. The solutions with high volume ratios of ethylene glycol/water (e.g., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) have relatively higher viscosities and lower dielectric constants, thus the solubility of the precipitate precursor and the diffusion rate of reactive species are effectively decreased. In addition, larger contents of ethylene glycol may improve the nucleation of crystals.35 These factors result in the high initial nucleation density of the precipitate precursor and subsequent two-dimensional growth,36,37 responsible for the formation of the product films with the nanosheet array structure. As water content in the solvothermal reaction solutions becomes larger, the solubility of the precipitate precursor and the diffusion rate of reactive species increase, leading to the low nucleation density of crystals. The easy diffusion capabilities of reactive species facilitate the oriented attachment of primary crystals on the nucleation sites due to dipole–dipole interactions.38–40 Consequently, a vertically aligned Co3O4 nanowire array on nickel foam is obtained in the solution with a low ethylene glycol/water volume ratio (1
9) have relatively higher viscosities and lower dielectric constants, thus the solubility of the precipitate precursor and the diffusion rate of reactive species are effectively decreased. In addition, larger contents of ethylene glycol may improve the nucleation of crystals.35 These factors result in the high initial nucleation density of the precipitate precursor and subsequent two-dimensional growth,36,37 responsible for the formation of the product films with the nanosheet array structure. As water content in the solvothermal reaction solutions becomes larger, the solubility of the precipitate precursor and the diffusion rate of reactive species increase, leading to the low nucleation density of crystals. The easy diffusion capabilities of reactive species facilitate the oriented attachment of primary crystals on the nucleation sites due to dipole–dipole interactions.38–40 Consequently, a vertically aligned Co3O4 nanowire array on nickel foam is obtained in the solution with a low ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59). While in the solution with a medium ethylene glycol/water volume ratio (1
59). While in the solution with a medium ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29), the unique 3D hierarchically porous Co3O4 network film, which is a mixed structure of nanowires and nanosheets, is obtained. As the content of ethylene glycol in the solvothermal reaction solutions increases, the morphology of Co3O4 undergoes the transformation from 1D nanowire to 2D nanosheet, demonstrating the controllability of its morphology and dimension.
29), the unique 3D hierarchically porous Co3O4 network film, which is a mixed structure of nanowires and nanosheets, is obtained. As the content of ethylene glycol in the solvothermal reaction solutions increases, the morphology of Co3O4 undergoes the transformation from 1D nanowire to 2D nanosheet, demonstrating the controllability of its morphology and dimension.
 |
| | Fig. 6 Schematic illustration for the growth mechanism of various nanostructured Co3O4 films on nickel foam substrate. | |
The as-synthesized Co3O4 films on nickel foam are directly used as the electrodes for electrochemical tests without the addition of any ancillary material, and the related results are exhibited in Fig. 7. The cyclic voltammetry (CV) curves of various Co3O4 film electrodes are illustrated in Fig. 7a. The shape of the CV curves clearly reveals the pseudocapacitive behavior of the Co3O4 film electrodes. The CV curves of the Co3O4 film electrodes present two couples of strong redox peaks in the potential window of 0 to 0.5 V, corresponding to the following surface redox reactions11,25
| | |
Co3O4 + OH− + H2O ↔ 3CoOOH + e−
| (11) |
| | |
CoOOH + OH− ↔ CoO2 + H2O + e−
| (12) |
 |
| | Fig. 7 Electrochemical properties of various Co3O4 film electrodes. (a) Cyclic voltammetry (CV) curves of various Co3O4 film electrodes at a scan rate of 5 mV s−1. (b) Initial galvanostatic charge/discharge profiles of various Co3O4 film electrodes at a current density of 2 mA cm−2. (c) Typical galvanostatic charge/discharge profiles of the WE29 electrode at various current densities. (d) Capacitances of the WE29 electrode at different current densities. (e) Cycle performance of the WE29 electrode at a current density of 5 mA cm−2 (4.348 A g−1). (f) Ragone plot of the WE29 electrode. | |
It is noted in Fig. 7a that the 3D hierarchically porous Co3O4 network film (WE29) electrode shows a much larger redox peak area than the other two Co3O4 film electrodes (WE9 and WE59), demonstrating its enhanced pseudocapacitive performance.
Fig. 7b gives the galvanostatic charge/discharge curves of various Co3O4 film electrodes in 6 M KOH solution at a current density of 2 mA cm−2. The WE29 electrode delivers a larger areal capacitance value (3.08 F cm−2) that WE9 (2.18 F cm−2) and WE59 (1.72 F cm−2) electrodes, which is consistent with the CV results. Fig. 7c exhibits the charge/discharge curves of the WE29 electrode at various current densities. All the curves show one couple of obvious charge and discharge plateaus, indicating a typical pseudocapacitance behavior. No obvious internal resistance drop is observed at the beginning of the discharge, indicating a low internal resistance for the WE29 electrode. As shown in Fig. 7d, the capacitances of the WE29 electrode at the current densities of 1 mA cm−2 (0.870 A g−1), 2 mA cm−2 (1.739 A g−1), 3 mA cm−2 (2.609 A g−1), 5 mA cm−2 (4.348 A g−1), 10 mA cm−2 (8.696 A g−1) and 20 mA cm−2 (17.391 A g−1) are 3.24 F cm−2 (2817 F g−1), 3.08 F cm−2 (2678 F g−1), 2.97 F cm−2 (2583 F g−1), 2.76 F cm−2 (2400 F g−1), 2.54 F cm−2 (2209 F g−1) and 2.24 F cm−2 (1948 F g−1), respectively. The specific capacitance at 17.391 A g−1 is as much as 69.2% of that at 0.870 A g−1, demonstrating its excellent rate capability. It is found by comparing the results in Fig. 7c, d, S3 and S4a† that the WE29 electrode displays obviously larger capacitances than other Co3O4 film electrodes (WE1, WE9 and WE59) at all the investigated current densities. The cycling performance of the WE29 electrode at a current density of 5 mA cm−2 (4.348 A g−1) is illustrated in Fig. 7e. The electrode delivers a capacitance of 1.87 F cm−2 (1628 F g−1) after 3500 cycles, showing a capacitance retention of 69%. As shown in Fig. 7e and S4b,† the WE29 electrode exhibits much higher capacitances than the other Co3O4 film electrodes (WE1, WE9 and WE59) during the whole electrochemical cycling. The specific power densities and specific energy densities at different current densities can be obtained from the galvanostatic charge/discharge curves in Fig. 7c.15 The Ragone plot (specific energy density vs. specific power density) is presented in Fig. 7f. As the charge/discharge current density increases from 0.870 to 17.391 A g−1, the energy density of the WE29 electrode decreases from 47.93 to 33.14 Wh kg−1, whereas the corresponding power density increases from 0.15 to 3.04 kW kg−1. The WE29 electrode shows a relatively large specific energy density of 33.14 Wh kg−1 at a high power density of 3.04 kW kg−1.26
The EIS measurements are performed to understand the electrochemical kinetics of the Co3O4 film electrodes with various microstructures. Fig. 8a shows the Nyquist plots of the three Co3O4 film electrodes (WE9, WE29 and WE59) at open circuit states. All the Nyquist plots are composed of one capacitive loop at high frequencies and an inclined line at low frequencies. The high-frequency capacitive loop can be attributed to the faradaic charge transfer resistance (Rct) in parallel with the double-layer capacitance (CPE1).15,21 The straight line in low-frequency region results from the mass transport limit of reactive species (W1). The high-frequency intercept of the capacitive loop on the real axis indicates the ohmic resistance (Rs) including the ionic resistance of electrolyte, the intrinsic resistance of the active material and the contact resistance at the active material/current collector interface. The diameter of the capacitive loop corresponds to the Rct value. The equivalent circuit in accordance with the above Nyquist plots is presented in Fig. 8b,15,41 and the fitted parameter values are shown in Table 1. It can be seen from Fig. 8a and Table 1 that the Rs, Rct and W1 values of the WE29 electrode are significantly lower than those of the WE9 and WE59 electrodes, and the CPE1 value of the WE29 electrode is much larger than the corresponding values of the other two electrodes. The larger CPE1 value shows the increased effectively electrochemical interface area of the WE29 electrode. The lower impedance (small values of Rs, Rct and W1) means the higher electrochemical activity of the WE29 electrode, which is generally agreement with the electrochemical results in Fig. 7.
 |
| | Fig. 8 (a) Nyquist plots of various Co3O4 film electrodes at open circuit states. (b) Corresponding equivalent circuit. | |
Table 1 Parameter values obtained by the fitting of the Nyquist plots in Fig. 8a
| Sample |
Rs (Ω) |
Rct (Ω) |
CPE1 (μF cm−2) |
n |
W1 (Ω cm−2) |
| WE9 |
0.128 |
0.01 |
0.015 |
0.878 |
0.405 |
| WE29 |
0.066 |
0.008 |
0.041 |
0.9 |
0.15 |
| WE59 |
0.168 |
0.021 |
0.011 |
0.834 |
0.502 |
Compared to Co3O4 based electrode materials recently reported for supercapacitors (Table S1†), the as-synthesized 3D hierarchically porous Co3O4 network film electrode (WE29) shows larger capacitances, excellent rate capability and good electrochemical cycling stability, which can be attributed to its unique structure characteristics. The hierarchically porous network architecture can provide easy access of the surfaces to liquid electrolyte, thus effectively increasing electrochemical interface area and offering more active sites for the pseudocapacitive reactions. The ultrathin Co3O4 nanosheets may shorten proton diffusion paths within the hydroxide/oxide solid phase, thereby enhancing the pseudocapacitive reactions.15,19,20 The 3D hierarchically porous Co3O4 network film with a mixed structure of nanowires and interconnected ultrathin nanosheets provides an ideal pathway for electrons. The distinct improvements in both electronic and ionic conductivities are mainly responsible for the larger specific capacitances and excellent rate capability of the Co3O4 network film electrode. The in situ formed free-standing structure not only has a close combination with nickel foam substrate, but also eliminates the need for binders and conductive additives required in a normal pasted electrode.31 This plays an important role in ensuring the good electrochemical cycling stability of the Co3O4 network film electrode. In addition, the hierarchically porous network architecture can buffer against the local volume change associated with the oxidation/reduction reaction processes, partially responsible for the good cycling stability of the electrode. The above main points contribute to the prominent capacitance performance of the 3D hierarchically porous Co3O4 network film electrode.
Conclusions
We have successfully fabricated different nanostructured Co3O4 films on nickel foam substrate by a facile solvothermal method. It is demonstrated that the morphologies and dimensions (1D nanowire to 2D nanosheet) of the Co3O4 films can be effectively tuned by tailoring the solvent compositions in the solvothermal reaction solutions. A possible effect mechanism of solvent composition on the morphologies of Co3O4 films is proposed. The 3D hierarchically porous Co3O4 network film synthesized in the solvothermal reaction solution with a medium ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29) shows prominent pseudocapacitive performance, such as large specific capacitance, excellent rate capability, good cyclic stability and enhanced energy density. This is mainly attributed to its unique 3D hierarchically porous network architecture and ultrathin Co3O4 nanosheets. These attractive results may open up the opportunity of developing the morphology-controlled nanomaterials for high-performance electrochemical energy storage and conversion systems.
29) shows prominent pseudocapacitive performance, such as large specific capacitance, excellent rate capability, good cyclic stability and enhanced energy density. This is mainly attributed to its unique 3D hierarchically porous network architecture and ultrathin Co3O4 nanosheets. These attractive results may open up the opportunity of developing the morphology-controlled nanomaterials for high-performance electrochemical energy storage and conversion systems.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21373182 and 51174176).
References
- X. Y. Lang, A. Hirata, T. W. Fujita and M. Chen, Nat. Nanotechnol., 2011, 6, 232–236 CrossRef CAS PubMed.
- Z. N. Yu, L. Tetard, L. Zhai and J. Thomas, Energy Environ. Sci., 2015, 8, 702–730 CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- C. Feng, J. F. Zhang, Y. D. Deng, C. Zhong, L. Liu and W. B. Hu, RSC Adv., 2015, 5, 42055–42062 RSC.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- C. G. Liu, Z. N. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS PubMed.
- M. G. Jeong, K. Zhou, S. Cherevko, W. J. Kim and C. H. Chung, J. Power Sources, 2013, 244, 806–811 CrossRef CAS.
- A. B. Yuan, X. L. Wang, Y. Q. Wang and J. Hu, Energy Convers. Manage., 2010, 51, 2588–2594 CrossRef CAS.
- S. S. Zhu, P. P. Zhang, L. Chang, Y. Zhong, K. Wang, H. B. Shao, J. M. Wang, J. Q. Zhang and C. N. Cao, Phys. Chem. Chem. Phys., 2016, 18, 8529–8536 RSC.
- S. Wang, T. Wang, Y. Shi, G. Liu and J. P. Li, RSC Adv., 2016, 6, 18465–18470 RSC.
- F. Y. Zhou, Q. L. Liu, J. J. Gu, W. Zhang and D. Zhang, J. Power Sources, 2015, 273, 945–953 CrossRef CAS.
- X. L. Hu, H. H. Huang, J. B. Zhang, J. J. Shi, S. F. Zhu and N. Su, RSC Adv., 2015, 5, 99899–99906 RSC.
- Y. H. Liu, W. Yu, L. Hou, G. H. He and Z. H. Zhu, RSC Adv., 2015, 5, 75105–75110 RSC.
- T. Ouyang, K. Cheng, S. Y. Kong, K. Ye, Y. Y. Gao, D. F. Zhang, G. L. Wang and D. X. Cao, RSC Adv., 2015, 5, 36059–36065 RSC.
- X. Dai, D. Chen, H. Q. Fan, Y. Zhong, L. Chang, H. B. Shao, J. M. Wang, J. Q. Zhang and C. N. Cao, Electrochim. Acta, 2015, 154, 128–135 CrossRef CAS.
- H. Jiang, C. Z. Li, T. Sun and J. Ma, Chem. Commun., 2012, 48, 2606–2608 RSC.
- K. Wang, J. Y. Huang and Z. X. Wei, J. Phys. Chem. C, 2010, 114, 8062–8067 CAS.
- J. P. Liu, J. Jiang, C. W. Cheng, H. X. Li, J. X. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076–2081 CrossRef CAS PubMed.
- X. H. Wang, S. W. Yao, X. X. Wu, Z. J. Shi, H. X. Sun and R. H. Que, RSC Adv., 2015, 5, 17938–17944 RSC.
- C. Z. Yuan, L. Yang, L. R. Hou, L. F. Shen, X. G. Zhang and X. W. Lou, Energy Environ. Sci., 2012, 5, 7883–7887 CAS.
- M. M. Yao, Z. H. Hu, Z. J. Xu and Y. F. Liu, J. Alloys Compd., 2015, 644, 721–728 CrossRef CAS.
- R. B. Rakhi, W. Chen, D. Cha and H. N. Alshareef, Nano Lett., 2012, 12, 2559–2567 CrossRef CAS PubMed.
- B. Wang, T. Zhu, H. B. Wu, R. Xu, J. S. Chen and X. W. Lou, Nanoscale, 2012, 4, 2145–2149 RSC.
- D. L. Ji, J. H. Li, L. M. Chen, D. Zhang, T. Liu, N. Zhang, R. Z. Ma, G. Z. Qiu and X. H. Liu, RSC Adv., 2015, 5, 41627–41630 RSC.
- R. Silva, G. M. Pererira, D. Voiry, M. Chhowalla and T. Asefa, RSC Adv., 2015, 5, 49385–49391 RSC.
- B. R. Sankapal, H. B. Gajare, S. S. Karade and D. P. Dubal, RSC Adv., 2015, 5, 48426–48432 RSC.
- H. W. Che and A. F. Liu, J. Mater. Sci.: Mater. Electron., 2015, 26, 4097–4104 CrossRef CAS.
- F. Manteghi, S. H. Kazemi, M. Peyvandipour and A. Asghari, RSC Adv., 2015, 5, 76458–76463 RSC.
- M. J. Deng, F. L. Huang, I. W. Sun, W. T. Tsai and J. K. Chang, Nanotechnology, 2009, 20, 175602 CrossRef PubMed.
- C. Feng, J. F. Zhang, Y. He, C. Zhong, W. B. Hu, L. Liu and Y. D. Deng, ACS Nano, 2015, 9, 1730–1739 CrossRef CAS PubMed.
- Y. G. Li, B. Tan and Y. Y. Wu, J. Am. Chem. Soc., 2006, 128, 14258–14259 CrossRef CAS PubMed.
- S. L. Xiong, C. Z. Yuan, X. G. Zhang, B. J. Xi and Y. T. Qian, Chem.–Eur. J., 2009, 15, 5320–5326 CrossRef CAS PubMed.
- J. Jiang, J. P. Liu, X. T. Huang, Y. Y. Li, R. M. Ding, X. X. Ji, Y. Y. Hu, Q. B. Chi and Z. H. Zhu, Cryst. Growth Des., 2010, 10, 70–75 CAS.
- R. Xu and H. C. Zeng, J. Phys. Chem. B, 2003, 107, 12643–12649 CrossRef CAS.
- Z. H. Ibupoto, K. Khun, J. Lu and M. Willander, Appl. Phys. Lett., 2013, 102, 103701 CrossRef.
- L. J. Zhao, H. J. Zhang, Y. Xing, S. Y. Song, S. Y. Yu, W. D. Shi, X. M. Guo, J. H. Yang, Y. Q. Lei and F. Cao, Chem. Mater., 2008, 20, 198–204 CrossRef CAS.
- L. F. Duan, S. S. Jia and L. J. Zhao, Mater. Res. Bull., 2010, 45, 373–376 CrossRef CAS.
- M. Talebi-Esfandarani and O. Savadogo, J. Appl. Electrochem., 2015, 45, 245–251 CrossRef CAS.
- H. I. Chen and H. Y. Chang, Colloids Surf., A, 2004, 242, 61–69 CrossRef CAS.
- J. Xu, J. Cai, J. M. Wang, L. Y. Zhang, Y. Q. Fan, N. Zhang, H. Zhou, D. Chen, Y. Zhong, H. Q. Fan, H. B. Shao, J. Q. Zhang and C. N. Cao, Electrochem. Commun., 2012, 25, 119–123 CrossRef CAS.
- T. Y. Wei, C. H. Chen, K. H. Chang, S. Y. Lu and C. C. Hu, Chem. Mater., 2009, 21, 3228–3233 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of the Co3O4 film (WE1), XRD pattern of Co(CO3)0.5(OH)·0.11H2O precursor, typical galvanostatic charge/discharge profiles of various Co3O4 film electrodes, areal capacitances at different current densities and cycle performances of various Co3O4 film electrodes, and comparison of the pseudocapacitive performances. See DOI: 10.1039/c6ra08117g |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29), shows a markedly enhanced pseudocapacitive performance. The specific capacitances of the Co3O4 network film electrode at the current densities of 0.870 and 17.391 A g−1 are 2817 and 1948 F g−1, respectively, revealing its large specific capacitance and excellent rate capability. Furthermore, the Co3O4 network film electrode exhibits good electrochemical cycling stability with a specific capacitance of 1628 F g−1 after 3500 cycles at a current density of 4.348 A g−1. The prominent pseudocapacitive performance of the Co3O4 network film electrode can be attributed to its unique structural characteristics. The as-synthesized 3D hierarchically porous Co3O4 network film with excellent pseudocapacitive performance demonstrates promising potential as a high-performance electrode for supercapacitors.
29), shows a markedly enhanced pseudocapacitive performance. The specific capacitances of the Co3O4 network film electrode at the current densities of 0.870 and 17.391 A g−1 are 2817 and 1948 F g−1, respectively, revealing its large specific capacitance and excellent rate capability. Furthermore, the Co3O4 network film electrode exhibits good electrochemical cycling stability with a specific capacitance of 1628 F g−1 after 3500 cycles at a current density of 4.348 A g−1. The prominent pseudocapacitive performance of the Co3O4 network film electrode can be attributed to its unique structural characteristics. The as-synthesized 3D hierarchically porous Co3O4 network film with excellent pseudocapacitive performance demonstrates promising potential as a high-performance electrode for supercapacitors.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 1
9, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and 1
29 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59. Prior to the synthesis, Ni foam (10 mm × 45 mm in rectangular shape) was treated in 2 M HCl solution for 10 min to remove the NiO surface layer, and ultrasonically cleaned in deionized water and acetone for 10 min, respectively. The reaction solution was vigorously stirred at room temperature. The as-prepared pink-colored homogeneous solution was then transferred into a 45 mL Teflon-lined stainless steel autoclave. After that, a piece of Ni foam with an exposed area of 10 × 20 mm2 (other section was protected from solution contamination by uniformly coating with a polytetrafluoroethylene tape) was then placed at an angle against the Teflon-lined wall of the stainless steel autoclave and immersed into the reaction solution. The sealed autoclave was maintained at 120 °C for 5 h in an electric oven. After synthesis, the autoclave was naturally cooled to room temperature. The substrate with the surface layer was rinsed with distilled water and ethanol, respectively. After dried at 60 °C for 10 h, the film sample was annealed at 250 °C in air for 3 h in a Muffle furnace. Careful weighing revealed that the loading masses of Co3O4 in various film samples were in the range of 0.9–1.16 mg cm−2. In the following discussions, the Co3O4 films synthesized in the ethylene glycol/water mixed solvents with volume ratios of 1
59. Prior to the synthesis, Ni foam (10 mm × 45 mm in rectangular shape) was treated in 2 M HCl solution for 10 min to remove the NiO surface layer, and ultrasonically cleaned in deionized water and acetone for 10 min, respectively. The reaction solution was vigorously stirred at room temperature. The as-prepared pink-colored homogeneous solution was then transferred into a 45 mL Teflon-lined stainless steel autoclave. After that, a piece of Ni foam with an exposed area of 10 × 20 mm2 (other section was protected from solution contamination by uniformly coating with a polytetrafluoroethylene tape) was then placed at an angle against the Teflon-lined wall of the stainless steel autoclave and immersed into the reaction solution. The sealed autoclave was maintained at 120 °C for 5 h in an electric oven. After synthesis, the autoclave was naturally cooled to room temperature. The substrate with the surface layer was rinsed with distilled water and ethanol, respectively. After dried at 60 °C for 10 h, the film sample was annealed at 250 °C in air for 3 h in a Muffle furnace. Careful weighing revealed that the loading masses of Co3O4 in various film samples were in the range of 0.9–1.16 mg cm−2. In the following discussions, the Co3O4 films synthesized in the ethylene glycol/water mixed solvents with volume ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 1
9, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and 1
29 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 were designated as WE1, WE9, WE29 and WE59, respectively.
59 were designated as WE1, WE9, WE29 and WE59, respectively.





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.
29.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. A vertically aligned array of Co3O4 nanowires with a diameter of ∼100 nm is synthesized in the solution with a low ethylene glycol/water volume ratio (1
9. A vertically aligned array of Co3O4 nanowires with a diameter of ∼100 nm is synthesized in the solution with a low ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59), as shown in Fig. 3e and f. The Co3O4 nanowires separately stem from nickel foam substrate, and converge on various points of the top sections, leading to the formation of a bundle-like structure. For the Co3O4 film synthesized in the solution with a medium ethylene glycol/water volume ratio (1
59), as shown in Fig. 3e and f. The Co3O4 nanowires separately stem from nickel foam substrate, and converge on various points of the top sections, leading to the formation of a bundle-like structure. For the Co3O4 film synthesized in the solution with a medium ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29), as shown by the SEM images in Fig. 3c and d a unique Co3O4 network structure is observed, which is formed by the interconnected ultrathin nanosheets on the top surface.
29), as shown by the SEM images in Fig. 3c and d a unique Co3O4 network structure is observed, which is formed by the interconnected ultrathin nanosheets on the top surface.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) have relatively higher viscosities and lower dielectric constants, thus the solubility of the precipitate precursor and the diffusion rate of reactive species are effectively decreased. In addition, larger contents of ethylene glycol may improve the nucleation of crystals.35 These factors result in the high initial nucleation density of the precipitate precursor and subsequent two-dimensional growth,36,37 responsible for the formation of the product films with the nanosheet array structure. As water content in the solvothermal reaction solutions becomes larger, the solubility of the precipitate precursor and the diffusion rate of reactive species increase, leading to the low nucleation density of crystals. The easy diffusion capabilities of reactive species facilitate the oriented attachment of primary crystals on the nucleation sites due to dipole–dipole interactions.38–40 Consequently, a vertically aligned Co3O4 nanowire array on nickel foam is obtained in the solution with a low ethylene glycol/water volume ratio (1
9) have relatively higher viscosities and lower dielectric constants, thus the solubility of the precipitate precursor and the diffusion rate of reactive species are effectively decreased. In addition, larger contents of ethylene glycol may improve the nucleation of crystals.35 These factors result in the high initial nucleation density of the precipitate precursor and subsequent two-dimensional growth,36,37 responsible for the formation of the product films with the nanosheet array structure. As water content in the solvothermal reaction solutions becomes larger, the solubility of the precipitate precursor and the diffusion rate of reactive species increase, leading to the low nucleation density of crystals. The easy diffusion capabilities of reactive species facilitate the oriented attachment of primary crystals on the nucleation sites due to dipole–dipole interactions.38–40 Consequently, a vertically aligned Co3O4 nanowire array on nickel foam is obtained in the solution with a low ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59). While in the solution with a medium ethylene glycol/water volume ratio (1
59). While in the solution with a medium ethylene glycol/water volume ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29), the unique 3D hierarchically porous Co3O4 network film, which is a mixed structure of nanowires and nanosheets, is obtained. As the content of ethylene glycol in the solvothermal reaction solutions increases, the morphology of Co3O4 undergoes the transformation from 1D nanowire to 2D nanosheet, demonstrating the controllability of its morphology and dimension.
29), the unique 3D hierarchically porous Co3O4 network film, which is a mixed structure of nanowires and nanosheets, is obtained. As the content of ethylene glycol in the solvothermal reaction solutions increases, the morphology of Co3O4 undergoes the transformation from 1D nanowire to 2D nanosheet, demonstrating the controllability of its morphology and dimension.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29) shows prominent pseudocapacitive performance, such as large specific capacitance, excellent rate capability, good cyclic stability and enhanced energy density. This is mainly attributed to its unique 3D hierarchically porous network architecture and ultrathin Co3O4 nanosheets. These attractive results may open up the opportunity of developing the morphology-controlled nanomaterials for high-performance electrochemical energy storage and conversion systems.
29) shows prominent pseudocapacitive performance, such as large specific capacitance, excellent rate capability, good cyclic stability and enhanced energy density. This is mainly attributed to its unique 3D hierarchically porous network architecture and ultrathin Co3O4 nanosheets. These attractive results may open up the opportunity of developing the morphology-controlled nanomaterials for high-performance electrochemical energy storage and conversion systems.



