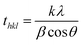Enhanced white light emission from a Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 nano-crystalline phosphor via Li+ doping
R. S. Yadav,
R. V. Yadav,
A. Bahadur and
S. B. Rai*
Department of Physics, Banaras Hindu University, Varanasi 221 005, India. E-mail: sbrai49@yahoo.co.in; Fax: +91-542-2369889; Tel: +91-542-230-7308
First published on 20th May 2016
Abstract
This paper reports white light emission from a Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 nano-crystalline phosphor synthesized through a solution combustion method. The structural measurements reveal the crystalline nature of the synthesized samples. The Tm3+/Yb3+ co-doped Na4ZnW3O12 sample gives intense blue emission due to the 1G4 → 3H6 transition whereas the Ho3+/Yb3+ co-doped Na4ZnW3O12 sample gives intense green and red emissions due to the 5F4/5S2 → 5I8 and 5F5 → 5I8 transitions, respectively, on excitation with 976 nm. When these three ions viz. Tm3+, Ho3+ and Yb3+; are co-doped in Na4ZnW3O12 the sample gives intense upconverted white light. The as-synthesized sample emits larger emission intensity on annealing at higher temperature. Addition of Li+ in the co-doped phosphor further enhances the emission intensity of white light up to two times and the CIE coordinates of the white light are (0.30, 0.41), which is close to the standard white light (0.33, 0.33). The enhancement in the emission intensity has been discussed due to changes in the local crystal field and reduction in the optical quenching centers. Thus, this nano-crystalline phosphor can be a suitable candidate for white light in various optical applications.
1. Introduction
The rare earth activated upconversion (UC) based inorganic optical materials have attracted much attention for their matchless efficiency to convert low energy near infrared (NIR) photons to high energy photons of visible (Vis) or ultraviolet (UV) light. They can be used as a luminescent label in biological systems for imaging and bio-detection purposes.1–4 The generation of rare earth doped UC white light materials has been fascinating in recent years. The white light can be obtained by mixing primary colors viz. red, green and blue in definite proportions or mixing of a primary and complementary colors or a broad continuum in the visible region. The rare earth ions are very rich in producing the primary and complementary colors efficiently. These ions have abundant energy levels and many of them are meta-stable in nature and play a very important role in these emissions.5–7 The UC based white light emitting materials have several advantages over conventional white light sources because of cost effectiveness, requirement of low energy for excitation, long life span, brightness and eco-friendly behavior.8–11The UC materials basically consist of three parts, as the host, activator and the sensitizer. In these materials, the Yb3+ ions are generally used as sensitizer in order to enhance the efficiency of the activators. It has large absorption cross section for 976 nm wavelength, which efficiently transfers its energy to the activator ion. On the other hand, the Tm3+ and Ho3+ ions are well known activators, whose emission intensity can be enhanced many folds in the presence of Yb3+ ion and emit blue, green and red colors thereby resulting white light. The white light emission from Tm3+/Yb3+/Ho3+ have been widely investigated by various workers in molybdate and tungstate host matrices.12–15 These workers observed the blue emission from Tm3+/Yb3+ co-doped system while green and red emissions from Ho3+/Yb3+ co-doped system, which collectively produced white light. Xia et al. have reported white light from Tm3+/Yb3+/Ho3+ co-doped Na0.5Gd0.5WO4 host synthesized by high temperature solid state reaction method and deduced that Ln3+-doped rare earth tungstate matrix has potential applications for lighting and displays.14 Recently, Cho et al. have achieved white light from these ions in CaWO4 host synthesized by a modified citrate complex method using microwave irradiation.15 It has also been noticed from these studies that the rare earth activated tungstates are chemically and physically stable and possess broad optical transparency from visible to NIR regions. In fact, the tungstate based compounds have been extensively investigated due to their unique optical properties.14–18 We have used Na4ZnW3O12 as a phosphor host and the optical properties of none of the activators have been reported in this host. It has been also observed that the optical properties from any of the rare earth ions are not yet reported in this host to our knowledge. There are various approaches to synthesize the highly luminescent UC based tungstate materials but there is no report found to synthesize this phosphor through solution combustion method.
The efficiency of luminescent materials can be enhanced further by co-doping an impurity element in it. Li+ ion is an alkali element and has been widely studied by various workers.19–24 It has been observed that Li+ ion affects the local crystal field symmetry around the activator ions. It has lower ionic radii and can enter easily into the materials to influence the local symmetry. As a result, it can occupy interstitial or substitutional sites in different materials and improve the crystallinity of the materials, which enhances the photoluminescence (PL) intensity significantly. In some cases, it is noticed that it reduces the optical quenching centers present in the sample, which further enhances the PL intensity of the materials.25–30 It has also been reported that the doping of ions with smaller ionic radii creates shrinkage in the lattice dimension thereby enhances the PL intensity appreciably.31
In the present work, we have synthesized Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 nano-crystalline phosphor for the first time through solution combustion method. Urea was used as an organic fuel (reducing agent) for combustion. The structural characterizations have been carried out using XRD, SEM and TEM measurement techniques. The photoluminescence properties of the synthesized samples have been studied using 976 nm laser radiation. The phosphor emits intense white light on excitation with 976 nm. It is found that addition of Li+ ions further enhances the emission intensity of the white light upto two times.
2. Experimental
2.1 Synthesis of the sample
The Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 nano-crystalline phosphor has been synthesized through solution combustion method. The Tm2O3, Yb2O3, Ho2O3, ZnO and Na2WO4·2H2O were taken as starting materials. Urea has been used as an organic fuel for combustion. The composition used are given as follows:| 60ZnO + (40 − x − y − z)Na2WO4·2H2O + xTm2O3 + yYb2O3 + zHo2O3 | (i) |
| 60ZnO + (40 − x − y − z − p)Na2WO4·2H2O + xTm2O3 + yYb2O3 + zHo2O3 + pLi2O | (ii) |
The stoichiometric ratios of Tm2O3, Yb2O3, Ho2O3 and ZnO (99.99% pure) were dissolved in 5 ml of nitric acid and then diluted slowly with de-ionized water. The diluted solutions were mixed by vigorous stirring. The Na2WO4·2H2O dissolved in distilled water was added to this solution with a constant stirring. Finally, urea was added to the solution as an organic fuel (reducing agent). The solution thus obtained was stirred at a constant temperature 60 °C for 4 hours. This was resulted a gel of the sample. The gel was then placed in a closed furnace maintained at 600 °C. It was found that an auto-ignition took place within few minutes and a white powder was obtained. This powder is termed as the as-synthesized nano-phosphor.
For preparation of Li+ incorporated co-doped nano-crystalline phosphor, the Li2O and other materials were dissolved in the nitric acid as mentioned above and the same process was repeated. The final product thus obtained was grinded in an agate mortar to convert it into fine powder. The powder samples were annealed in batches at 1200 °C for five hours separately to enhance their structural and optical properties.
2.2 Characterization
X-ray diffraction patterns of the as-synthesized and the annealed samples without and with Li+ ion were recorded using Cu Kα radiation (λ = 0.15406 nm) from a RINT/DMAX 2200H/PC (Rigaku, Japan) machine with 2° min−1 scan speed at room temperature. Data from International Centre for Diffraction (ICDD) was used to identify the crystallite phase of the as-synthesized and the annealed samples. The microstructure of the sample was studied using a scanning electron microscopy (SEM) with JEOL-TM Model JSM 5410 unit and transmission electron microscopy (TEM) with Tecnai 20G2, Philips unit. The PL spectra of different samples were monitored using 976 nm radiation from a diode laser and iHR320, Horiba Jobin Yvon, spectrometer attached with PMT was used to record the spectra. The lifetime of Tm3+ and Ho3+ ions in the different samples were measured by chopping the continuous wave of 976 nm laser using 150 MHz digital oscilloscope (Model No. HM 1507, Hameg Instruments).3. Results and discussion
3.1 Structural characterizations
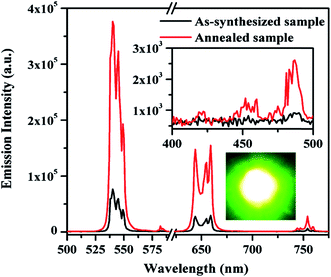
The XRD measurements are the primary way in distinguishing the phase, crystallinity and average crystallite size of the synthesized samples. The XRD patterns of the as-synthesized and the annealed Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 and its Li+ added annealed samples have been recorded in the range of 20–80° and they are shown in Fig. 1(a). It is evident from the figure that all the samples are crystalline. The crystallinity of the as-synthesized sample increases on annealing at higher temperature, which is clearly verified from the figure. The diffraction patterns of all the samples were confirmed with the help of international center for diffraction data (ICDD), which match well with JCPDS file no. 30-1269. The average crystallite size (thkl) for three intense lattice planes are calculated using Debye–Scherrer equationwhere λ is the wavelength of the X-ray radiation, β is the FWHM of the diffraction peak, θ is the angle of diffraction and k is a constant equal to 0.90.
The average crystallite size is found to be 18 and 38 nm for the as-synthesized and the annealed nano-crystalline phosphor samples, respectively. This confirms that the annealed sample is more crystalline than the as-synthesized one. When Li+ ion is added to the sample the crystallite size slightly increases to 40 nm and the peak is shifted towards higher angle side (see inset figure). The inset in Fig. 1(a) shows a shift in most intense peak position on addition of Li+ ion. The shifting of peaks towards higher angle side confirms the shrinkage in the lattice structure. This is due to the fact that Li+ ion with smaller ionic radii (76 pm) replaces Na+ ion (102 pm), which creates shrinkage in lattice dimensions. Similarly, Chung et al. have reported the effect of Li+ ion in CaMoO4 host and observed that due to smaller ionic radii, Li+ ion can be inserted into the host at substitutional sites of Ca2+ for its lower concentration whereas at interstitial sites for higher concentration and it can break or distort the local crystal field around the rare earth ions.28 This enhances the fluorescence intensity of the sample appreciably. The reduction in cell size and thereby lattice parameter can be a favorable approach to enhance the emission intensity of the samples. This is also similar to the case of CaMoO4:Ho3+/Yb3+/Mg2+ phosphor. It has been observed that the effect of Mg2+ ion substitution in the structure of CaMoO4 results an enhancement of fluorescence intensity. It is concluded that Mg2+ ion with lower ionic radii substitutes Ca2+ ion with higher ionic radii from the host and creates shrinkage in lattice dimensions.31 Thus, in our case Li+ substitution affects the lattice structure and creates shrinkage in lattice dimension.
Fig. 1(b) shows the TEM micrograph of Li+ doped Tm3+/Yb3+/Ho3+:Na4ZnW3O12 nano-crystalline phosphor sample. The particles are spherical in shape and agglomerated with each other. The average size of the particle is ∼80 nm. The inset in Fig. 1(b) shows the selected area electron diffraction (SAED) pattern, which reveals the single crystalline nature of the sample.
The SEM micrographs of the annealed (at 1200 °C/5 h) Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 nano-crystalline phosphors without and with Li+ ion are shown in Fig. 2(a) & (b). The particles in both the cases are seen to be spherical in shape and agglomerated with each other. This is due to the organics used in the synthesis, which could not completely remove from samples during combustion. When Li+ ion is added to the Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 sample the particle size seems slightly larger (see Fig. 2(b)).31
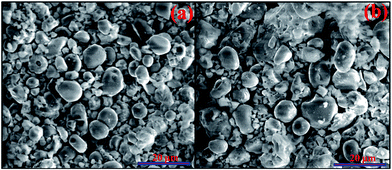 | ||
| Fig. 2 SEM micrographs of the annealed (a) Tm3+/Yb3+/Ho3+ and (b) its Li+ doped Na4ZnW3O12 nano-crystalline phosphors. | ||
3.2 Optical characterizations
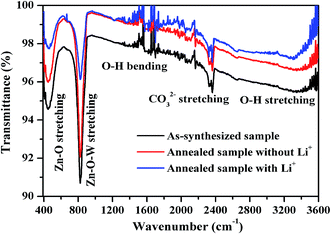 | ||
| Fig. 3 FTIR spectra of the as-synthesized and the annealed Tm3+/Yb3+/Ho3+ co-doped nano-crystalline phosphors without and with Li+ ion. | ||
The as-synthesized sample contains various impurities such as CO32− (2372 cm−1), OH− (3415 cm−1) etc.6 These impurities act as optical quenching centers and are responsible for poor UC emission intensity. This creates non-radiative transitions, which degrades the emission intensity. These impurities get reduced significantly when the as-synthesized sample is annealed at higher temperatures. The effect of reduction of these quenching centers can be clearly documented from the emission spectra. The spectra also contain vibrational bands at 450 and 823 cm−1, which are assigned due to stretching vibrations of Zn–O and Zn–O–W, respectively.34–37 It is clear from the figure that when Li+ ion is added to the sample the impurities present in the sample further reduced considerably.27 This is more favourable for optical properties and results a further enhancement in the emission intensity. The peaks of the Zn–O and Zn–O–W vibrational bands remains unchanged even after annealing and reduction in the intensity for these bands is probably due to the replacement of Na+ by Li+ ion from the sample.
The different mechanisms involved in absorption of incident photons, energy transfer (ET) and upconversion emissions can be easily understood using energy level diagram. The schematic energy level diagram for the Tm3+/Yb3+ co-doped sample is shown in Fig. 5.
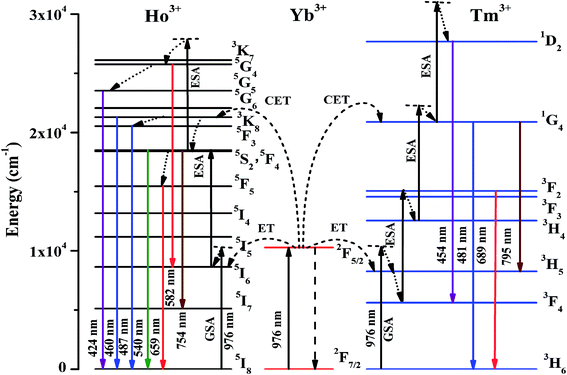 | ||
| Fig. 5 Schematic energy level diagram for Ho3+/Yb3+/Tm3+ ions and different transitions on excitation with 976 nm. | ||
It is well known that Tm3+ ion absorbs 976 nm photon very weakly and results poor emission intensity. However, the emission intensity can be enhanced many folds in the presence of Yb3+ ions. In fact, the Yb3+ ions have large absorption cross section for 976 nm. On excitation, the Yb3+ ions absorb the incident 976 nm photons and are promoted from the ground state (2F7/2) to the excited state (2F5/2). On de-excitation, these ions transfer their excitation energy to the Tm3+ ions in the ground state (3H6). As a result, Tm3+ ions are promoted to 3H5 state through ground state absorption (GSA) due to energy transfer upconversion (ETU). The excited Tm3+ ions in 3H5 state relax non-radiatively to 3F4 state and absorb another 976 nm photon. These ions are then promoted to 3F2 state through excited state absorption (ESA), which relaxes non-radiatively to the 3H4 state. The ions in 3H4 state further absorb 976 nm photons and are promoted to 1G4 state through ESA. Simultaneously, the Yb3+ ions also transfer their energy to Tm3+ ions in ground state through cooperative energy transfer (CET) process, which easily promote Tm3+ ions to 1G4 excited state. Thus, the ions in 1G4 state are further excited to the 1D2 state through ESA. The transitions from 1D2, 1G4 and 3F2 states to the lower states emit radiations of different wavelengths such as 454, 481, 689 and 795 nm.6,32,33
The input pump power versus emission intensity measurements have been carried out in order to verify the involvement of number of photons in upconversion emission. A dual logarithmic plot of input pump power versus emission intensity of Tm3+/Yb3+ co-doped Na4ZnW3O12 nano-crystalline phosphor for 481 nm transition is given in Fig. 6. The UC emission intensity of the sample is directly proportional to the nth power of input pump power and it is given as
| IUC ∝ Pn |
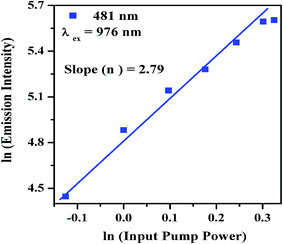 | ||
| Fig. 6 Dual logarithmic plot of input pump power versus emission intensity of the annealed Tm3+/Yb3+ co-doped Na4ZnW3O12 nano-crystalline phosphor sample for 481 nm transition. | ||
The value of the slope (n) has been calculated and is found to be 2.79, which is close to 3. This indicates that the 1G4 level of Tm3+ ion is populated by the absorption of three NIR photons.6,38 The CIE coordinates for Tm3+/Yb3+ co-doped Na4ZnW3O12 nano-crystalline phosphor has been calculated and it is found to be (0.17, 0.14), which is found in the blue region. The synthesized sample results intense blue color perception to the naked eye.
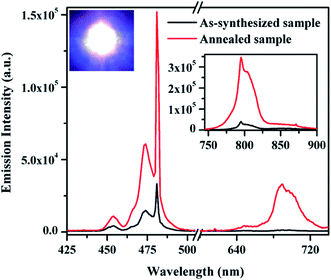
Since the Ho3+ ions have large number of meta-stable states and can be easily excited to get larger fluorescence. The Yb3+ ions easily transfer their excitation energy to the levels of Ho3+ ions and enhance the emission intensity significantly. The Yb3+ ions absorb 976 nm photons and are excited to (2F5/2) state. These ions on de-excitation easily transfer their energy to the Ho3+ ions in the ground state (5I8). As a result, the Ho3+ ions are excited to 5I6 state through ground state absorption (GSA) due to ETU. The Ho3+ ions in this state further absorb 976 nm photons and are promoted to the 5F4/5S2 excited states through ESA thereby emitting photons of 540 and 582 nm. The ions in this state relax non-radiatively to the 5F5 state and emit a photon of 659 nm. The ions in the 5F4 state further absorb another photons through CET and are promoted to 3K7 state through ESA populating 5G4, 5G5, 3K8 and 5F3 states, which on de-excitation emit different photons.39–42 The excitation and emission processes can be easily understood by schematic energy level diagram. The schematic energy level diagram for Ho3+/Yb3+ ions are shown in Fig. 5, which shows the involvement of different mechanisms such as GSA, ESA, ETU and CET in the excitation processes.
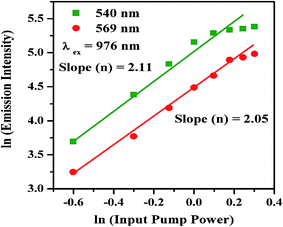
The power dependence measurements have been performed to confirm the number of photons involved in UC emission. The UC emission intensity of the nano-crystalline phosphor has been measured at different laser pump power. A dual logarithmic plot of input pump power versus emission intensity for Ho3+/Yb3+ co-doped Na4ZnW3O12 nano-crystalline phosphor for 540 and 659 nm transitions is shown in Fig. 8. The values of the slopes (n) are found to be 2.11 and 2.05 for 540 and 659 nm transitions, respectively.39 This indicates that the 5S2 and 5F5 levels of Ho3+ ion are populated by absorption of two photons.
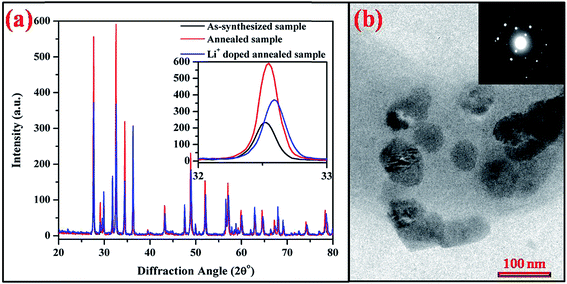
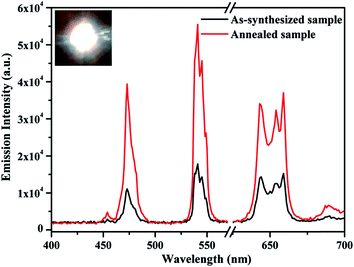
The UC emission spectra of the as-synthesized and the annealed (0.7 mol%) Tm3+/(0.5 mol%) Ho3+/(3.0 mol%) Yb3+ co-doped Na4ZnW3O12 nano-crystalline phosphors on excitation with 976 nm are shown in Fig. 9. When the concentration of Tm3+ ions varies as 0.3, 0.5, 0.7 and 0.9 mol% the color emitted by the sample varies from greenish white to white and finally turns to bluish white. The change in the emitted color can be easily seen even with naked eyes. This clearly suggests that the concentration of the rare earth ions greatly affect the color of emitted light. Herein, it has been also observed that the annealed sample further shows an enhancement in the emission intensity.
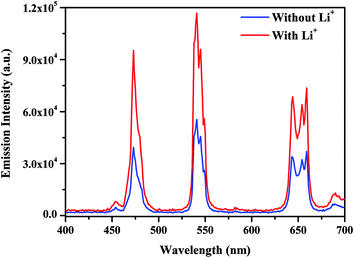 | ||
| Fig. 10 UC emission spectra of the annealed (0.7 mol%) Tm3+/(0.5 mol%) Ho3+/(3.0 mol%) Yb3+ co-doped and its (5 mol%) Li+ doped Na4ZnW3O12 nano-crystalline phosphors on excitation with 976 nm. | ||
It is worth noting that due to smaller ionic radii Li+ can be easily inserted into Na4ZnW3O12 crystal lattice. Li+ (0.76 Å) ion easily substitutes the Na+ (102 Å) ion from the crystal and results shrinkage in the crystal lattice. This leads a change in local crystal field around rare earth ions and the rare earth ions are expected close to each other. Therefore, the rare earth ions are strongly excited and results larger emission intensity for the Li+ doped sample compared to without Li+ doped sample. The effect of Li+ on the emission intensity of white light in Y2O3 host has been studied by Bai et al. and they observed that the presence of Li+ enhances the emission intensity of white light emission to a great extent. They have also explained that the addition of Li+ ion modifies the local symmetry and thereby changes the crystal structure.25 In our case, Li+ due to lower ionic radii occupies most of the sites of Na+ in Na4ZnW3O12 host and creates shrinkage in the lattice of the host, which is favorable for better emission intensity.31 Similarly, the effect of Li+ ion has been studied by Chung et al. in CaMoO4 host and deduced that the modification in the local crystal field by Li+ ion around the rare earth ions leads to a larger emission intensity.28
On the other hand, Pandey et al. have studied the effect of Li+ ion in Tb3+, Yb3+ co-doped Y2O3 phosphor and proved that Li+ co-doping reduces the optical quenching centers thereby enhances the emission intensity.27 It is also evident from Fig. 3 that the presence of Li+ reduces the optical quenching centers, which further improves the emission intensity of white light. Therefore, Li+ affects the local crystal field around rare earth ions and reduces the optical quenching centers thereby enhances the emission intensity upto two times. Hence, Li+ acts as sensitizer for the Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 sample.
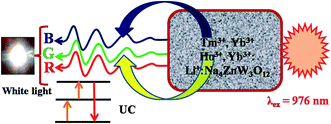
The color emitted by the phosphor can be identified by human eyes, which is the best tool to confirm the nature of colors. Another way to check the color of the emitted light is by calculating the CIE (Commission internationale de l'e'clairage) color coordinates in the two dimensions. The CIE color coordinates for Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 sample in presence of Li+ is shown in Fig. 11. The CIE color coordinates has been calculated and found to be (0.30, 0.41), which is close to the standard CIE coordinates (0.33, 0.33) of white light.8 Thus, this phosphor sample can be useful as white light emitting material for various optical applications. The mechanism of white light emission from Li+ incorporated Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 nano-crystalline phosphor is summarized in a working model and is shown in Fig. 12.
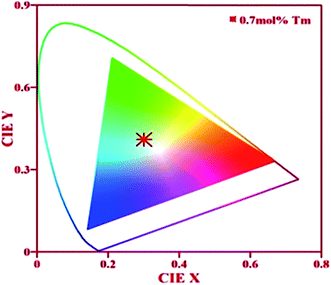 | ||
| Fig. 11 CIE diagram of the annealed (0.7 mol%) Tm3+/(0.5 mol%) Ho3+/(3.0 mol%) Yb3+ co-doped Na4ZnW3O12 nano-crystalline phosphor in presence of (5 mol%) Li+ on excitation with 976 nm. | ||
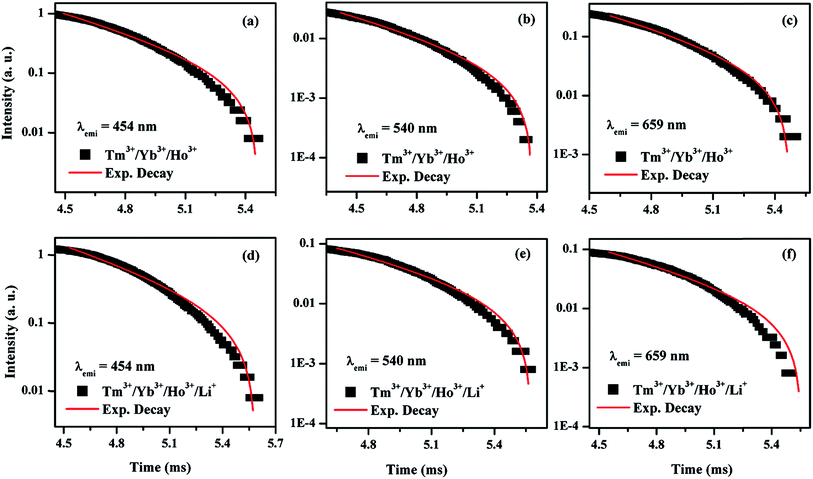
| Sample | τ (μs), λemi = 454 nm | τ (μs) | |
|---|---|---|---|
| λemi = 540 nm | λemi = 659 nm | ||
| Tm3+/Yb3+/Ho3+ | 463 | 492 | 465 |
| Tm3+/Yb3+/Ho3+/Li+ | 532 | 539 | 525 |
4. Conclusion
The Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 nano-crystalline phosphor has been synthesized through solution combustion method. The structural measurements reveal crystalline nature of the synthesized sample. The Tm3+/Yb3+ co-doped Na4ZnW3O12 sample gives intense blue (481 nm) color due to the 1G4 → 3H6 transition whereas Ho3+/Yb3+ co-doped Na4ZnW3O12 sample gives intense green (540 nm) and red (659 nm) colors due to the 5F4/5S2 → 5I8 and 5F5 → 5I8 transitions, respectively on excitation with 976 nm. When these three viz. Tm3+, Ho3+ and Yb3+; ions are co-doped in Na4ZnW3O12, the sample emits intense upconverted white light. The color emitted by the sample is tunable with the concentration of Tm3+ ion and changes from greenish blue to white and finally turns to bluish white. The sample annealed at higher temperature emits larger emission intensity. The presence of Li+ in the co-doped phosphor further enhances the emission intensity of white light upto two times with its CIE coordinates (0.30, 0.41), which is close to the standard white light (0.33, 0.33). The enhancement is due to change in the local crystal field and reduction in the optical quenching centers. Thus, the Li+ incorporated Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 nano-crystalline phosphor is expected to be a white light emitting material for various optical applications.Acknowledgements
The authors wish to acknowledge to Prof. O. N. Srivastava, Department of Physics, Banaras Hindu University, Varanasi for extending XRD, SEM and TEM measurement facilities. We also acknowledge the financial assistance from ‘UGC’ – ‘India’ (Grant No. F. 40-423/2011(SR)) and ‘DST’ – ‘India’ (Grant No. SR/S2/LOP-023/2012).References
- D. Vennerberg and Z. Lin, Sci. Adv. Mater., 2011, 3, 26 CrossRef.
- F. Auzel, C. R. Seances Acad. Sci., Ser. B, 1966, 263, 819 Search PubMed.
- V. Ovsyankin and P. P. Feofilov, JETP Lett., 1966, 4, 317 Search PubMed.
- M. Lin, Y. Zho, S. Wang, M. Liu, Z. Duan, Y. Chen, F. Li, F. Xu and T. Lu, Biotechnol. Adv., 2012, 30, 1551 CrossRef CAS PubMed.
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed.
- R. S. Yadav, R. K. Verma and S. B. Rai, J. Phys. D: Appl. Phys., 2013, 46, 275101 CrossRef.
- L. Han, L. Zhao, J. Zhang, Y. Wang, L. Guo and Y. Wang, RSC Adv., 2013, 3, 21824 RSC.
- Y. Gao, M. Fan, Q. Fang and F. Yang, New J. Chem., 2014, 38, 146 RSC.
- F. Wang and X. Liu, J. Am. Chem. Soc., 2008, 130, 5642 CrossRef CAS PubMed.
- Z. Yu, Q. Yang, C. Xu and Y. Liu, Mater. Res. Bull., 2009, 44, 1576 CrossRef CAS.
- L. Guo, Y. Wang, J. Zhang and P. Dong, J. Electrochem. Soc., 2011, 158, 225 CrossRef.
- J. H. Chung, J. H. Ryu, S. W. Mhin, K. M. Kim and K. B. Shim, J. Mater. Chem., 2012, 22, 3997 RSC.
- J. Sun, B. Xue and H. Du, Mater. Sci. Eng., B, 2013, 178, 822 CrossRef CAS.
- Z. Xia, W. Zhou, H. Du and J. Sun, Mater. Res. Bull., 2010, 45, 1199 CrossRef CAS.
- H. Cho, S. M. Hwang, J. B. Lee, D. H. Ka, T. W. Kim, B. S. Lee, J. Y. Lee, J. I. Lee and J. H. Ryu, Trans. Nonferrous Met. Soc. China, 2014, 24, 134 CrossRef.
- J. Wang, X. Jing, C. Yan, J. Lin and F. Liao, J. Lumin., 2006, 121, 57 CrossRef CAS.
- Q. Dai, H. W. Song, X. Bai and G. H. Pan, J. Phys. Chem. C, 2007, 111, 7586 CAS.
- J. Yu, K. Huang, L. Yuan and S. Feng, New J. Chem., 2015, 39, 6730 RSC.
- S. Bachir, K. Azuma, J. Kossanyi, P. Valat and J. C. R. Haret, J. Lumin., 1997, 75, 35 CrossRef CAS.
- Z. Zhou, T. Komori, T. Ayukawa, H. Yukawa, M. Morinaga, A. Koizumi and Y. Takeda, Appl. Phys. Lett., 2005, 87, 091109 CrossRef.
- Y. F. Bai, K. Yang, Y. X. Wang, X. R. Zhang and Y. L. Song, Opt. Commun., 2008, 281, 2930 CrossRef CAS.
- D. Uki, H. Ohnishi, T. Yamaguchi, Y. Takemori, A. Koizumi, S. Fuchi, T. Ujihara and Y. Taked, J. Cryst. Growth, 2007, 298, 69 CrossRef CAS.
- J. H. Jeong, K. S. Shim, H. K. Yang, J. S. Bae, B. K. Moon, S. S. Yi, J. H. Kim and Y. S. Kim, J. Lumin., 2007, 122, 87 CrossRef.
- Z. Y. Lu, F. F. He, P. C. Xu, Y. C. Teng and B. Wang, J. Wuhan Univ. Technol., 2008, 23, 20 CrossRef CAS.
- Y. F. Bai, Y. Wang, G. Peng, W. Zhang, Y. Wang, K. Yang, X. Zhang and Y. Song, Opt. Commun., 2009, 282, 1922 CrossRef CAS.
- H. Guo, N. Dong, M. Yin, W. Zhang, L. Lou and S. Xia, J. Phys. Chem. B, 2004, 108, 19205 CrossRef CAS.
- A. Pandey, V. K. Rai and K. Kumar, Spectrochim. Acta, Part A, 2014, 118, 619 CrossRef CAS PubMed.
- J. H. Chung, S. Y. Lee, K. B. Shim, S. Y. Kweon, S. C. Ur and J. H. Ryu, Appl. Phys. A, 2012, 108, 369 CrossRef CAS.
- K. A. Hyeon, S. H. Byeon, J. C. Park, D. K. Kim and K. S. Suh, Solid State Commun., 2000, 115, 99 CrossRef CAS.
- J. C. Park, H. K. Moon, D. K. Kim, S. H. Byeon, B. C. Kim and K. S. Suh, Appl. Phys. Lett., 2000, 77, 2162 CrossRef CAS.
- R. Dey, A. Kumari, A. K. Soni and V. K. Rai, Sens. Actuators, B, 2015, 210, 581 CrossRef CAS.
- D. Gao, X. Zhang, H. Zheng, P. Shi, L. Li and Y. Ling, Dalton Trans., 2013, 42, 1834 RSC.
- A. Kumari, A. Pandey, R. Dey and V. K. Rai, RSC Adv., 2014, 4, 21844 RSC.
- R. P. Jia, G. X. Zhang, Q. S. Wu and Y. P. Ding, Mater. Lett., 2007, 61, 1793 CrossRef CAS.
- S. M. Samuel, J. Koshy, A. Chandran and K. C. George, Indian J. Pure Appl. Phys., 2010, 48, 703 Search PubMed.
- K. A. Alim, V. A. Fonoberov and A. A. Balandin, Appl. Phys. Lett., 2005, 86, 053103 CrossRef.
- P. Siriwong, T. Thongtem, A. Phuruangrat and S. Thongtem, CrystEngComm, 2011, 13, 1564 RSC.
- Q. Lu, A. Li, F. Y. Guo, L. Sun and L. C. Zhao, Nanotechnology, 2008, 19, 145701 CrossRef PubMed.
- V. Kumar, P. Rani, D. Singh and S. Chawla, RSC Adv., 2014, 4, 36101 CAS.
- S. Huang, X. Zhang, L. Wang, L. Bai, J. Xu, C. Li and P. Yang, Dalton Trans., 2012, 41, 5634 RSC.
- G. H. Dieke, Spectra and Energy Levels of Rare Earth Ions in Crystals, Wiley-Interscience, New York, 1968 Search PubMed.
- R. V. Yadav, S. K. Singh and S. B. Rai, RSC Adv., 2015, 5, 26321 RSC.
- R. S. Yadav, R. V. Yadav, A. Bahadur, T. P. Yadav and S. B. Rai, Mater. Res. Express, 2016, 3, 036201 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |