DOI:
10.1039/C6RA06731J
(Paper)
RSC Adv., 2016,
6, 51757-51767
Adsorptive recovery of palladium(II) from aqueous solution onto cross-linked chitosan/montmorillonite membrane
Received
14th March 2016
, Accepted 21st May 2016
First published on 23rd May 2016
Abstract
A cross-linked chitosan/montmorillonite (CCTS-M) membrane was prepared successfully with a glutaraldehyde cross-linker, and then was characterized by FTIR, XRD, SEM/EDX, TG/DTG and XPS. The materials were used for removing palladium(II) from aqueous solutions. The effect of variable parameters including initial pH, contact time, initial concentration, and temperature were investigated. The results showed that the maximum capacity of the membrane was 193 mg g−1 at pH 2. This article not only described the adsorption behavior but also suggested isotherms, the kinetics data were best modeled using pseudo-second-order model, and the Langmuir isotherm model could fit the experimental data well. The thermodynamic parameters ΔG° (<0) and ΔH° (>0) indicated that the adsorption was a spontaneous and endothermic process. FTIR and XPS techniques confirmed that the –NH3+, –NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– functional groups in the CCTS-M membrane played prominent roles in the adsorption process. Our results revealed that the membrane could be a promising adsorbent for the recovery of palladium.
N– functional groups in the CCTS-M membrane played prominent roles in the adsorption process. Our results revealed that the membrane could be a promising adsorbent for the recovery of palladium.
1 Introduction
Palladium, one of the most important platinum group metals (PGM), has been widely applied in many fields, such as jewelry, electronic components, catalysts and dental alloys, due to its favorable physical and chemical properties.1,2 However, palladium as a new pollutant affects the environment to an increasing degree, especially in the technical use of catalysts containing active palladium metal.2–4 This metal has generally accumulated along motorways, in vegetation or on soil surfaces, and then concerns have arisen with respect to the health risks generated by the possible inhalation of dusts and contamination through food and water.5 Therefore, effective separation and purification of palladium from waste materials, e.g. spent catalysts, as well as its removal from the environment are of special interest.5–7
Traditional methods are available for recovery of palladium from aqueous solutions, such as precipitation, solvent extraction,8,9 ion-exchange10 and adsorption. Among these techniques, adsorption is one of the promising techniques, owing to its higher enrichment factor, faster kinetics, minimal organic diluents utilization and less waste accumulation. Recently, modifications of chelating resins, chemically-modified activated carbon, nanotubes, biomass and functionalized silica gel have been widely studied to overcome the problem of low capacities of palladium recovery.11–13 However, many fine-particle adsorbents sometimes would cause certain disadvantages, because of difficulty in recycling, easy loss of the adsorbent, and unfavorable hydrodynamic properties.14 Fortunately, membrane processes are considered to be better alternatives and more appropriate for the removal of metal ions.15–17 Because it reduced obviously mass transfer resistance compared to column processes, resulting in fast binding behavior with high linear velocity of the mobile phase.14 Therefore, membrane processes can be used for the recovery of palladium and resolve a critical determinant for cost-effective operation of traditional technology. Recently, more efficient and cheaper membrane adsorbents had been paid more significant attention. Chitosan is a biopolymer produced by alkaline N-deacetylation of chitin, and widely found in the exoskeletons of shellfish and crustaceans.18,19 Meanwhile, it has been already used in membrane processes, due to its excellent film-forming properties, highly reactive amino groups as well as its nontoxicity and biodegradability.18,20–22 But its disadvantages is that poor mechanical properties (easy shrinking and swelling) and limited chemical stability (high solubility in acidic media). Accordingly, crosslinking and organic–inorganic hybridization approaches, e.g. crosslinking with glutaraldehyde, epichlorohydrin and blending with nanoclay, have commonly been carried out to avoid drawbacks.22,23 In recent years, various polymer/clay composites have displayed unique properties in terms of improved tensile strength, flexibility, and flexural endurance.24 Montmorillonite, a kind of layered silicates, is well-known as a support material for chitosan composites polymer on account of the higher specific surface area and better cationic exchange capacity.25,26 Thus it is considered to be potential for lamellar expansion, which can improve the mechanical properties and water slug resistance at low addition levels.27 A large number of the chitosan/montmorillonite composites are applied in engineering or medical materials, but less for its environmental properties and precious metal recovery.
In the present work, the organic–inorganic hybridization membranes of chitosan and Na-montmorillonite (CCTS-M) were prepared using glutaraldehyde as a cross-linker to produce a new adsorbent, and were further analyzed by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), field emission scanning electron microscope with energy dispersive X-ray (SEM/EDX), and thermogravimetric (TG) analysis. The effects of solution pH, contact time, initial Pd(II) concentration and temperature on the adsorption properties of (CCTS-M) membranes were investigated. Moreover, the adsorption mechanism is further discussed through X-ray photoelectron spectroscopy (XPS).
2 Materials and methods
2.1 Materials
Chitosan (shrimp shells based, ≥95% deacetylated, viscosity 100–200 mPa s) was purchased from Aladdin. Na-montmorillonite (Na-MMT) was obtained from Chifeng, Inner Mongolia Autonomous Region. Atomic adsorption spectrometry standard solutions of 1000 mg L−1 Pd(II) were obtained from Beijing NCS Analytical Instruments Co. Ltd. Glutaraldehyde, acetic acid, and sodium hydroxide were supplied by Guangzhou Chemical Reagent Factory. Other chemical agents used were all analytical grade and all solutions were prepared with distilled water.
2.2 Preparation
Cross-linked chitosan/montmorillonite (CCTS-M) membranes were prepared according to the previous studies.23 In brief, chitosan powder (2.0 g) was dissolved in 40 mL of acetic acid (2 wt%). Na-montmorillonite (Na-MMT) (0.1 g) was dispersed in 10 mL of acetic acid (2 wt%) by ultrasonication for 30 min, and then added to the chitosan solution slowly. In addition, 0.5 mL glutaraldehyde solution (2.5 wt%) was added into the above mixture. After vigorous stirring for 2 h, a homogeneous and stable chitosan/Na-MMT solution was obtained. The chitosan/Na-MMT solution was filtered and left standing for at least 30 min under vacuum to remove air bubbles, and the CCTS-M membrane was obtained by direct solvent evaporation in Petri dishes at 60 °C. The membrane was then immersed in 1 M NaOH solution for 6 h in order to neutralize the excess acetic acid. Finally, the membrane was rinsed and stored in distilled water at 4 °C. Pure chitosan membranes (CTS) were also prepared following the method mentioned above, but without using glutaraldehyde and Na-montmorillonite.
2.3 Characterizations
XRD patterns were measured by a BRUKER D8 ADVANCE diffractometer with flat-filtered Cu Kα radiation. FTIR (Thermo, Nicolet 6700) spectra of the materials were recorded at room temperature in the wave number range 650 to 4000 cm−1. SEM (ZEISS Ultra 55) images were collected to determine morphology of the samples, and an elemental analysis of the membrane was done by EDX spectroscopy (Oxford X-Max 50). TG was performed by a Perkin-Elmer thermogravimetric apparatus coupled to a thermobalance heated to 800 °C at a heating rate of 10 °C min−1 under a dry nitrogen atmosphere. The chemical analyses of the virgin and metal-loaded membranes were conducted by X-ray photoelectron spectroscopy (XPS) (ESCALAB 250, Thermo-VG Scientific), and the data were collected in binding energy forms and all of the binding energies were referenced to the neutral C 1s speak at 284.6 eV, in order to compensate for the surface charging effects.
2.4 Adsorption experiments
Batch adsorption experiments were carried out in a temperature-controlled shaker at 298 K. The batch reactor was a 50 mL flask, in which 20 mL of Pd(II) solution and 10 ± 1 mg of the membrane adsorbent were shaken at 200 rpm to ensure complete mixing. The effect of solution pH was investigated using 100 mg L−1 of the initial concentration in the pH range of 1–5 adjusted with 0.1 M HCl and NaOH solutions. The kinetic studies were conducted according to following methodology: Two different concentrations of solution (75 and 100 mg L−1), 100 mL each, was thoroughly mixed with 10 ± 1 mg membrane at pH 2. Aliquots were taken at time intervals for the analysis of residual concentration. Adsorption isotherm studies were conducted by varying the initial concentration from 25–300 mg L−1 at pH 2 and 298 K. The influence of temperature was studied under the following conditions: membrane adsorbent dose 10 ± 1 mg, pH value of 2, varying concentrations of 50–100 mg L−1, and temperature in the range of 293–313 K. After equilibrium, the residual metal concentration in the supernatant was determined by AAS (PinAAcle 900T PerkinElmer). The amount of Pd(II) adsorbed per unit mass of adsorbent at equilibrium (qe) was obtained using the following equation:| |
 | (1) |
where C0 and Ce are initial and equilibrium concentrations (mg L−1), M is the wet mass of membrane (g), and V is the volume of solution (L).
3 Results and discussions
3.1 Characterizations
3.1.1 XRD. Fig. 1a showed the XRD patterns of pure CTS, Na-MMT, and CCTS-M. The characteristic peaks of CTS appeared at around 2θ = 10.38°, 14.15°, 16.82°, 20.02° and 22.26° in agreement with a previous publication.28 The pattern of Na-MMT exhibited a distinctive diffraction peak at 7.13°, which corresponded to a d001 basal spacing of 1.23 nm. For the pattern of CCTS-M, it was obvious that Na-MMT caused significant changes in the peak intensity of CTS, which was accompanied by shifting of the d001 diffraction peak of MMT to a lower angle (4.99°), and increased in the basal spacing from 1.23 nm to 1.74 nm after Na-MMT interacted with chitosan. These results indicated the intercalation of CTS into the interlayer spacing of Na-MMT,29 and could be caused mainly by the ion exchange between Na+ ions of the Na-MMT and the positive charged amine groups of chitosan.30 In addition, the diminution of the Na-MMT peaks provided a strong evidence of intercalated structure where the chitosan chains were incorporated between the silicate layers.29 The addition of glutaraldehyde resulted in the decrease of chitosan crystallinity of CCTS-M membrane compared with that of CTS membrane in accordance with the previous studies.23 Besides, the disappearance of the peaks at 14.15° and 16.82° indicated the weakening of hydrogen bonding interactions in CTS, which implied that the chain alignments of chitosan became more disordered after the cross-linking reaction with glutaraldehyde. The changes in the XRD pattern suggested that a strong chemical bond could be formed in the CCTS-M membrane.
 |
| | Fig. 1 XRD patterns of pure CTS membrane, Na-MMT, and CCTS-M membrane (a), and FT-IR spectra of Na-MMT, pure CTS, CCTS-M and CCTS-M-Pd(II) (b). | |
3.1.2 FTIR. The FTIR spectra of Na-MMT, CTS, CCTS-M and CCTS-M after Pd(II) adsorption (CCTS-M-Pd) are shown in Fig. 1b. The FTIR spectrum of Na-MMT showed a band at 3618 cm−1, which is assigned to stretching vibration of the –OH group within the clay structure, and the band at 3451 cm−1 was ascribed to the –OH vibrations of water molecules. The strong bands observed in the range of 1160–1045 cm−1 referred to the characteristic stretching vibrations of the Si–O–Si moiety, which agreed with those in the literature.30 For CTS membrane, a broad intense band at around 3500–3200 cm−1, was ascribed to –OH and –NH2 stretching vibrations. The stretching vibrations of –CH bond in –CH2 and –CH3 groups appeared at 2930 cm−1 and 2845 cm−1, respectively. The band at 1664 cm−1 was associated with the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the amide group CONH– and the band at 1587 cm−1 was related to the deformation vibration of the amine group. The strong absorption band at around 1030 cm−1 was characteristic of pyranose rings of chitosan which matched well with the reported results.21 Compared to the CTS membrane, the spectrum of the CCTS-M membrane showed a combination of the characteristic absorptions of chitosan and Na-MMT groups. The –OH and Si–O–Si groups of Na-MMT (3618 cm−1 and 1032 cm−1) were also observed in the spectra of CCTS-M membranes, but these bands shifted to lower wavenumbers (3610 cm−1 and 1027 cm−1) in CCTS-M membranes, which were induced by the hydrogen bonding interaction between Na-MMT and chitosan. The intensity of the band at 1027 cm−1 increased, which might be because the C–O–C group vibration of CTS overlapped with the Si–O–Si stretching vibration of Na-MMT.29 Moreover, the new peak at 1650 cm−1 (C
O of the amide group CONH– and the band at 1587 cm−1 was related to the deformation vibration of the amine group. The strong absorption band at around 1030 cm−1 was characteristic of pyranose rings of chitosan which matched well with the reported results.21 Compared to the CTS membrane, the spectrum of the CCTS-M membrane showed a combination of the characteristic absorptions of chitosan and Na-MMT groups. The –OH and Si–O–Si groups of Na-MMT (3618 cm−1 and 1032 cm−1) were also observed in the spectra of CCTS-M membranes, but these bands shifted to lower wavenumbers (3610 cm−1 and 1027 cm−1) in CCTS-M membranes, which were induced by the hydrogen bonding interaction between Na-MMT and chitosan. The intensity of the band at 1027 cm−1 increased, which might be because the C–O–C group vibration of CTS overlapped with the Si–O–Si stretching vibration of Na-MMT.29 Moreover, the new peak at 1650 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and the shifting of peak (–NH2) by about 7 cm−1 can be attributed to the crosslinking reaction between glutaraldehyde and chitosan. In addition, the absorptions centered at about 3362 cm−1 and 3294 cm−1 reflected the amine groups in CCTS-M membrane. These FTIR results were comparable to the reports of glutaraldehyde-crosslinked chitosan,22,28 and indicated the formation of CCTS-M membrane. Significant changes of CCTS-M-Pd membrane were observed that the band at about 3294 cm−1 was related to –NH2 in unloaded membrane, and changed to 3223 cm−1 after adsorption of Pd(II) (Fig. 1b). The peak of Si–O–Si stretching vibration also shifted from 1027 cm−1 to 1038 cm−1. In addition, the –NH2 deformation vibration at 1593 cm−1 and the C
N) and the shifting of peak (–NH2) by about 7 cm−1 can be attributed to the crosslinking reaction between glutaraldehyde and chitosan. In addition, the absorptions centered at about 3362 cm−1 and 3294 cm−1 reflected the amine groups in CCTS-M membrane. These FTIR results were comparable to the reports of glutaraldehyde-crosslinked chitosan,22,28 and indicated the formation of CCTS-M membrane. Significant changes of CCTS-M-Pd membrane were observed that the band at about 3294 cm−1 was related to –NH2 in unloaded membrane, and changed to 3223 cm−1 after adsorption of Pd(II) (Fig. 1b). The peak of Si–O–Si stretching vibration also shifted from 1027 cm−1 to 1038 cm−1. In addition, the –NH2 deformation vibration at 1593 cm−1 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– stretching vibration at 1650 cm−1 were also observed to shift to 1577 cm−1 and 1623 cm−1 respectively, suggesting that a strong metal–ligand bond formed between Pd(II) and chelating groups involving the –NH2 and C
N– stretching vibration at 1650 cm−1 were also observed to shift to 1577 cm−1 and 1623 cm−1 respectively, suggesting that a strong metal–ligand bond formed between Pd(II) and chelating groups involving the –NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– groups in the CCTS-M membrane.
N– groups in the CCTS-M membrane.
3.1.3 SEM-EDX. SEM images of CTS, CCST-M and CCTS-M-Pd membranes are shown in Fig. 2. The CTS membrane had smooth surfaces and no obvious hole distribution (Fig. 2a). In the case of CCST-M membrane, rough surfaces with obvious uneven area and tiny pores were observed (Fig. 2b), which were in favor of the effective adsorption. After adsorption of Pd(II), CCTS-M-Pd membrane showed a more dense structure than the surface prior to adsorption (Fig. 2c). The apparent morphological changes were attributed to the interaction between the CCTS-M and Pd(II). Fig. 2d–f show the EDX data for these three membranes. The presence of Al and Si was observed in the EDX spectrum of CCTS-M membrane (Fig. 2e), which further confirmed the incorporation of Na-MMT into the CCTS-M membrane. The adsorption of Pd(II) onto CCTS-M membrane was also confirmed through the EDX spectral analysis (Fig. 2f). The distinct peaks of Pd and Cl were in the range of 2.5–3.5 keV, which was assumed that the Cl was involved in the adsorption process.
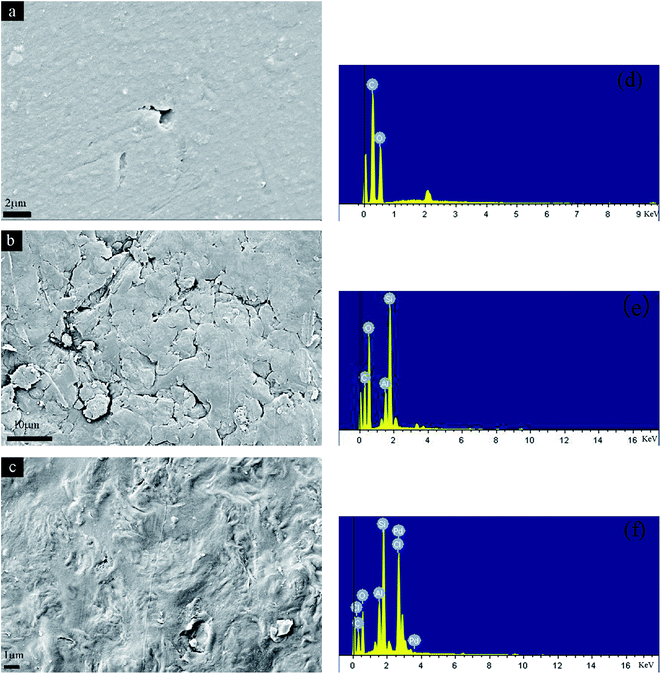 |
| | Fig. 2 SEM micrograph of pure CTS membrane (a), CCTS-M membrane (b), and CCTS-M-Pd membrane (c); EDX of pure CTS membrane (d), CCTS-M membrane (e), and CCTS-M-Pd membrane (f). | |
3.1.4 TG-DTG. The thermal stability of Na-MMT, pure CTS and CCTS-M membrane were investigated by TG analysis under nitrogen flow. Fig. 3a and b show the TG and DTG curves of these samples. Two weight losses for Na-montmorillonite sample at around 79 °C and 639 °C were observed, which were assigned to the adsorbed water on the surface linked by hydrogen bonds and the coordinated water in the interlamellar space, respectively. The results were similar to a previous investigation.29 Under nitrogen environment, there were two stages of nonoxidative degradation for the CTS and CCTS-M membranes. The first weight loss below 150 °C corresponded to the absorbed water molecules. These samples present similar amount of absorbed water (5 wt%), and the second stage of decomposition occurs between 250 °C and 330 °C, due to the degradation and deacetylation of chitosan.29 After heating to 800 °C, the maximum mass loss reached 55 wt% and 52 wt% for CTS and CCTS-M membranes, respectively. Moreover, decomposition temperatures were also obtained from the differentiated TG curves (Fig. 3b) in order to compare the thermal stabilities of CTS membranes and CCTS-M membranes. The results clearly indicated that CCTS-M membrane was more labile thermally than the pure CTS membrane, because of the synergetic effect of MMT platelets and CS on the thermal properties.
 |
| | Fig. 3 TGA (a) and DTG (b) of pure CTS membrane, Na-MMT and CCTS-M membrane. | |
3.2 Effect of pH
The pH of aqueous solution is an important factor that strongly affects the adsorption process. Fig. 4a showed that the effect of pH (1–5) on the adsorption of Pd(II) onto the CCTS-M membrane. It can be seen that Pd(II) adsorption increased from 35 to 126 mg g−1 with an increase in the pH from 1 to 2 and reached the maximum at pH 2. As the result of FTIR analysis, CCTS-M membrane contained a significant amount of –NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– groups. At lower pH, the CCTS-M membrane was positively charged due to protonation of the amino groups, while the Pd(II) existed mostly as an anionic complex, viz. PdCl42− in hydrochloric acid solution, leading to an electrostatic attraction between them. The large excess of chloride anions and the strong competition between anions (Cl−) and PdCl42− restricted metal adsorption. However, with the increase of pH from 1 to 2, this competition decreased due to decrease in the amount of Cl−1 from HCl and the adsorption of metal increased.11 The maximum adsorption of Pd(II) on the CCTS-M membrane was 126 mg g−1 occurred at pH 2. Then the adsorption capacity decreased to 56 mg g−1 with further increased in the pH to 5, which may be attributed to the formation of hydrolyzed chloropalladium complex, to make the Pd(II) ions unavailable in the adsorption process. Additionally, the H+ concentration is insufficient for chitosan –NH2 to form the NH3+ to capture Pd(II) at high pH values.4 A much higher pH was not tested because of the possibility of Pd precipitation as Pd(OH)2. Therefore, pH 2 was chosen as the optimal pH condition in the following experiments.
N– groups. At lower pH, the CCTS-M membrane was positively charged due to protonation of the amino groups, while the Pd(II) existed mostly as an anionic complex, viz. PdCl42− in hydrochloric acid solution, leading to an electrostatic attraction between them. The large excess of chloride anions and the strong competition between anions (Cl−) and PdCl42− restricted metal adsorption. However, with the increase of pH from 1 to 2, this competition decreased due to decrease in the amount of Cl−1 from HCl and the adsorption of metal increased.11 The maximum adsorption of Pd(II) on the CCTS-M membrane was 126 mg g−1 occurred at pH 2. Then the adsorption capacity decreased to 56 mg g−1 with further increased in the pH to 5, which may be attributed to the formation of hydrolyzed chloropalladium complex, to make the Pd(II) ions unavailable in the adsorption process. Additionally, the H+ concentration is insufficient for chitosan –NH2 to form the NH3+ to capture Pd(II) at high pH values.4 A much higher pH was not tested because of the possibility of Pd precipitation as Pd(OH)2. Therefore, pH 2 was chosen as the optimal pH condition in the following experiments.
 |
| | Fig. 4 Effect of pH on adsorption of Pd(II) (initial concentration: 100 mg L−1; t: 12 h; m: 10 ± 1 mg; temperature: 298 K) (a), effect of contact time and initial concentration on the adsorption of Pd(II) (pH: 2; m: 10 ± 1 mg; temperature: 298 K) (b), and the pseudo-second order kinetics model of adsorption of Pd(II) (c). | |
3.3 Adsorption kinetic
In order to investigate the adsorption equilibrium and the adsorption process rate, the effect of time on the Pd(II) adsorption was performed and the results were shown in Fig. 4b. Initially, the adsorption increased rapidly within the first 100 min, and then increased slowly until it reached the equilibrium in 300 min. The high adsorption rate in the beginning was probably due to the rapid interaction between Pd(II) ions and the abundant availability active sites on the external surface of the adsorbent; then with gradual occupancy of the sites, the decrease in site availability decreased the adsorption efficiency, which was reflected in the slower rate between 100 and 300 min. In order to evaluate the kinetic mechanism that controls the adsorption process, the adsorption data were treated with two simplified kinetic models, including a pseudo-first order kinetics model and a pseudo-second order kinetics model, which were expressed by eqn (2) and (3), respectively:31| |
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t qe − K1t
| (2) |
| |
 | (3) |
where qe and qt (mg g−1) are the adsorption capacities at equilibrium and at t (min), respectively, K1 (min−1) is the rate constant of the pseudo-first-order adsorption, while K2 (g mg−1 min−1) is the rate constant of the pseudo-second-order adsorption.
The linear correlation coefficient for the pseudo-first-order model was very low (R2 < 0.8), indicating that the pseudo-first-order model was only a modest fit for the adsorption process. However, the determination coefficient value for the pseudo-second-order adsorption model (Fig. 4c) (R2 = 0.9993) was higher and more close to unity. Besides, the calculated equilibrium adsorption capacity qe(cal) (169.49 mg g−1) based on the pseudo-second-order model agreed better with the experimental value qe(exp) (166.68 mg g−1), suggesting that the pseudo-second-order model was more suitable for describing the adsorption kinetics of Pd(II) onto CCTS-M membrane. The results indicated that the adsorption rate was controlled by chemical adsorption of Pd(II) onto the CCTS-M membrane.13
3.4 Adsorption isotherm
The equilibrium adsorption isotherm is fundamental to describe the interactive behavior between adsorbate and adsorbent, and is also important in designing an adsorption system. Isotherms were obtained by varying the initial concentration of Pd(II) from 25 to 300 mg L−1 and the results were shown in Fig. 5a. It was clear that the higher the initial concentration of Pd(II), the better for the its adsorption. The adsorption capacity of CCTS-M membrane increased from 42 to 193 mg g−1 with the increase of the Pd(II) concentration from 25 to 300 mg L−1. The Langmuir isotherm model was used to interpret the adsorption data due to the homogeneous surface of the adsorbent, and the Langmuir isotherm is represented by the following equation:13| |
 | (4) |
where Ce (mg L−1) is the equilibrium concentration of Pd(II) in the solution, Qe (mg g−1) is the amount of Pd(II) ion adsorbed at equilibrium, Qm (mg g−1) is the maximum adsorption capacity corresponding to monolayer coverage, and KL (L mg−1) is the Langmuir adsorption constant. The values of Qm and KL can be calculated from plotting Ce/Qe versus Ce. In order to determine whether the adsorption process was favorable or not, a dimensionless constant separation factor or equilibrium parameter, RL was defined according to the following equation:31,32| |
 | (5) |
where KL (L mg−1) is the Langmuir adsorption constant and C0 (mg L−1) is the initial Pd(II) concentration. The value of RL would indicate whether the isotherm was irreversible (RL = 0), favorable (0 < RL < 1), linear (RL = 1) or unfavorable (RL > 1).30
 |
| | Fig. 5 Effect of initial concentration on the adsorption of Pd(II) (pH: 2; m: 10 ± 1 mg; t: 300 min; temperature: 298 K) (a), Langmuir isotherm model (b) and Freundlich isotherm model for the adsorption of Pd(II) onto CCTS-M membrane (c) (pH: 2; m: 10 ± 1 mg; t: 300 min; temperature: 298 K), effect of temperature and initial concentration on the adsorption of Pd(II) (initial concentration: 50, 75 and 100 mg L−1; pH: 2; m: 10 ± 1 mg; t: 300 min) (d), and plot of free energy ΔG° and T (e). | |
The Freundlich isotherm model, which describes heterogeneous adsorption system, can be represented as follows:13
| |
 | (6) |
where the
Ce (mg L
−1) and
qe (mg g
−1) are the equilibrium concentration of Pd(
II) in solution and on CCTS-M membrane, respectively.
KF (mg g
−1) and
n are Freundlich constants, which were calculated from the slope and intercept of the plot of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus
qe versus ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce.
The calculated parameter values of the Langmuir and Freundlich equations were given in Fig. 5b and c, respectively. The experimental data fitted the Langmuir isotherm model better as indicated by the linear regression coefficients (R2 = 0.9847), whereas the lower for the Freundlich isotherm model (R2 = 0.9296), which indicated that the adsorption of Pd(II) by the CCTS-M membrane was homogenous at monolayer level. A reasonable explanation would be that Pd(II) in the aqueous solution was bound on the surface of CCTS-M membrane as a monolayer through chemical bonding with –NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–. The adsorption capacity of the CCTS-M membrane adsorbent was found to be 196.07 mg g−1 according to the fitted parameter of the Langmuir equation. Moreover, the RL value of 0.58, which was between 0 and 1, confirmed that the conditions were favorable for the adsorption of Pd(II) onto the CCTS-M membrane.13
N–. The adsorption capacity of the CCTS-M membrane adsorbent was found to be 196.07 mg g−1 according to the fitted parameter of the Langmuir equation. Moreover, the RL value of 0.58, which was between 0 and 1, confirmed that the conditions were favorable for the adsorption of Pd(II) onto the CCTS-M membrane.13
3.5 Adsorption thermodynamics
To understand the thermodynamic behavior of the adsorption of Pd(II) on the CCTS-M membrane, the effect of temperature (293–313 K) was further investigated and the results were shown in Fig. 5d. The adsorption capacity increased slowly with an increase in temperature. The thermodynamic parameters including Gibbs free energy change (ΔG°), enthalpy change (ΔH°) and entropy change (ΔS°) were calculated using the following equations:13| |
ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL KL
| (7) |
| |
 | (8) |
where KL is the equilibrium constant; R is the gas constant (8.314 J mol−1 K−1); T (K) is the absolute temperature; the values of ΔH° (kJ mol−1) and ΔS° (kJ mol−1 K−1) can be calculated from the slope and intercept of the linear plot of ΔG° versus T. The negative value of ΔG° indicated a spontaneous nature of the adsorption and the effectiveness of the interaction between Pd(II) and the membrane adsorbent. The positive value of ΔH° (7.61 kJ mol−1) showed that the adsorption process was endothermic as shown in Fig. 5d and e, and the adsorption capacity of adsorbent increased with the increase of temperature. The positive value of ΔS° (0.03 kJ mol−1 K−1) corresponded to an increase in randomness at the solid/solution interface with some structural changes in the adsorbate and the adsorbent during the adsorption of Pd(II) on the CCTS-M membrane.31
3.6 XPS analysis and adsorption mechanism
The XPS analysis was performed for CCTS-M and CCTS-M-Pd membranes in order to identify the adsorption mechanism of Pd(II) loaded onto CCTS-M membrane, and the results were presented in Fig. 6 and Table 1. The XPS wide scan spectra of CCTS-M membrane before and after Pd(II) adsorption showed that major peaks such as C 1s, O 1s and N 1s were present (Fig. 6a). A distinct peak was clearly observed for CCTS-M-Pd, which was assigned to Pd 3d and confirmed the adsorption of Pd(II) onto CCTS-M membrane. In addition, The Cl 2p peak appearing in the XPS spectra of CCTS-M-Pd also identified that the Cl atom participated in the process of adsorption, which was also consistent with the EDX results. The high resolution core-level spectra of O 1s, N 1s and Pd 3d of CCTS-M and CCTS-M-Pd were shown in Fig. 6b–f. The O 1s spectra can be resolved into three individual component peaks, which came from different functional groups and overlap with each other. The peaks of binding energy (BE) of 532.84, 531.81 and 533.66 eV were assigned to the oxygen atom in the forms of C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Si–O bonds, respectively.33 There were no obvious changes in the O 1s binding energies after Pd(II) adsorption (Fig. 6b and c), indicating that there were no or little chelating interactions between the groups containing oxygen and Pd(II).34 In the case of N 1s binding energy in high resolution XPS analysis, several peaks have been identified and their variations could be correlated with the changes in the chemistry of membrane after Pd(II) adsorption. The N 1s spectrum of CCTS-M showed three individual component peaks at 401.59 eV for –NH3+, 399.56 eV for –NH2 and 399.11 eV for –N
O and Si–O bonds, respectively.33 There were no obvious changes in the O 1s binding energies after Pd(II) adsorption (Fig. 6b and c), indicating that there were no or little chelating interactions between the groups containing oxygen and Pd(II).34 In the case of N 1s binding energy in high resolution XPS analysis, several peaks have been identified and their variations could be correlated with the changes in the chemistry of membrane after Pd(II) adsorption. The N 1s spectrum of CCTS-M showed three individual component peaks at 401.59 eV for –NH3+, 399.56 eV for –NH2 and 399.11 eV for –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, respectively (Fig. 6d). After adsorption, the N 1s binding energies of –NH2 and –N
C, respectively (Fig. 6d). After adsorption, the N 1s binding energies of –NH2 and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in CCTS-M-Pd were higher than that in CCTS-M, and shifted to 400.37 and 399.29 eV, respectively (Fig. 6e), due to donation of electrons from the N atoms of –NH2 and –N
C bonds in CCTS-M-Pd were higher than that in CCTS-M, and shifted to 400.37 and 399.29 eV, respectively (Fig. 6e), due to donation of electrons from the N atoms of –NH2 and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups to Pd(II), thereby indicating chelating interactions between these groups and Pd(II).35 As a consequence, the electron cloud density of the nitrogen atom was reduced, resulting in a higher observed binding energy peak. In addition, the peak at 401.73 eV assigned to –NH3+ increased significantly after Pd(II) adsorption, as seen in the Fig. 6e, possibly due to the formation of NH3+–(PdCl4)2−. The changes in the binding energies of –NH3+, –NH2 and –N
C groups to Pd(II), thereby indicating chelating interactions between these groups and Pd(II).35 As a consequence, the electron cloud density of the nitrogen atom was reduced, resulting in a higher observed binding energy peak. In addition, the peak at 401.73 eV assigned to –NH3+ increased significantly after Pd(II) adsorption, as seen in the Fig. 6e, possibly due to the formation of NH3+–(PdCl4)2−. The changes in the binding energies of –NH3+, –NH2 and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C indicated that N 1s was involved in the adsorption of Pd(II).36 These results accounted for the formation of CCTS-M–N⋯Pd complexes in the reactions of eqn (11)–(13). The high resolution Pd 3d spectrum of CCTS-M-Pd membrane was shown in Fig. 6f. The sample exhibited a Pd 3d5/2 peak belonging to the characteristic of divalent Pd at around 338 eV.36,37 It should be noted that the Pd 3d5/2 of CCTS-M-Pd exhibited two peaks at about 338.01 eV and 336.42 eV, indicating two different Pd environments: the peak at 336.42 eV was characteristic of the complexation formed between –N
C indicated that N 1s was involved in the adsorption of Pd(II).36 These results accounted for the formation of CCTS-M–N⋯Pd complexes in the reactions of eqn (11)–(13). The high resolution Pd 3d spectrum of CCTS-M-Pd membrane was shown in Fig. 6f. The sample exhibited a Pd 3d5/2 peak belonging to the characteristic of divalent Pd at around 338 eV.36,37 It should be noted that the Pd 3d5/2 of CCTS-M-Pd exhibited two peaks at about 338.01 eV and 336.42 eV, indicating two different Pd environments: the peak at 336.42 eV was characteristic of the complexation formed between –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/–NH2 groups and Pd(II); another peak at 338.01 eV corresponded to the Pd 3d5/2 electron binding energies of Pd(II).35 These can be ascribed to two complex ions for Pd in the adsorption process. Therefore, the possible adsorption mechanism can be described by eqn (10)–(13) and Fig. 7.
C/–NH2 groups and Pd(II); another peak at 338.01 eV corresponded to the Pd 3d5/2 electron binding energies of Pd(II).35 These can be ascribed to two complex ions for Pd in the adsorption process. Therefore, the possible adsorption mechanism can be described by eqn (10)–(13) and Fig. 7.| | |
CCTS-M–NH2 + H+ ↔ CCTS-M–NH3+
| (10) |
| | |
CCTS-M–NH3+ + PdCl42− ↔ PdCl42−(CCTS-M–NH3+)2
| (11) |
| |
CCTS-M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N + PdCl42− ↔ PdCl2(CCTS-M N + PdCl42− ↔ PdCl2(CCTS-M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2 + 2Cl− N)2 + 2Cl−
| (12) |
| | |
CCTS-M–NH2 + PdCl42− ↔ PdCl2(CCTS-M–N)2 + 2Cl−
| (13) |
 |
| | Fig. 6 The XPS spectra of CCTS-M and CCTS-M-Pd (a). The high-resolution core-level spectra of (b and c) O 1s, (d and e) N 1s for CCTS-M and CCTS-M-Pd. (f) The high-resolution core-level spectra of Pd 3d for CCTS-M-Pd. | |
Table 1 The binding energy of C 1s, O 1s, N 1s in CCTS-M and CCTS-M-Pd membrane
| XPS |
C 1s (eV) |
O 1s (eV) |
N 1s (eV) |
| CCTS-M |
286.41 |
532.88 |
399.41 |
| CCTS-M-Pd(II) |
286.45 |
532.89 |
401.66 |
399.35 |
 |
| | Fig. 7 Schematic representation of the adsorption mechanism of Pd(II). | |
4 Conclusions
In this paper, a novel organic–inorganic hybrid CCTS-M membrane was prepared with low cost chitosan and Na-MMT. This membrane adsorbent showed a high adsorption capacity for Pd(II) and its maximum adsorption capacity of Pd(II) at 298 K was 193 mg g−1 at pH 2. The kinetic and isotherm studies indicated that pseudo-second-order model and the Langmuir model better described the adsorption equilibrium of Pd(II) on CCTS-M membrane. The adsorption processes were accompanied by an increase in entropy and exhibit endothermic enthalpy values. The –NH3+, –NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– functional groups in CCTS-M membrane were confirmed to be involved in the Pd(II) adsorption process by XPS and FTIR techniques. These results are highly promising and suggest the CCTS-M membrane can be applied for recovery of palladium.
N– functional groups in CCTS-M membrane were confirmed to be involved in the Pd(II) adsorption process by XPS and FTIR techniques. These results are highly promising and suggest the CCTS-M membrane can be applied for recovery of palladium.
Acknowledgements
The authors acknowledge funding from the National Natural Science Foundation of China (No. 51508206 and 21006037), and Natural Science Foundation of Guangdong province (No. 2015A030310328 and 06300845).
References
- F. Bai, G. Ye, G. Chen, J. Wei, J. Wang and J. Chen, Sep. Purif. Technol., 2013, 106, 38–46 CrossRef CAS.
- B. R. Reddy, B. Raju, J. Y. Lee and H. K. Park, J. Hazard. Mater., 2010, 180, 253–258 CrossRef CAS PubMed.
- M. Mihaljevič, I. Galušková, L. Strnad and V. Majer, J. Geochem. Explor., 2013, 124, 212–217 CrossRef.
- M. R. Awual, M. A. Khaleque, Y. Ratna and H. Znad, J. Ind. Eng. Chem., 2015, 21, 405–413 CrossRef CAS.
- R. K. Sharma, A. Pandey, S. Gulati and A. Adholeya, J. Hazard. Mater., 2012, 209–210, 285–292 CrossRef CAS PubMed.
- Y. Leng, J. Xu, J. Wei and G. Ye, Chem. Eng. J., 2013, 232, 319–326 CrossRef CAS.
- R. Ruhela, K. Singh, B. S. Tomar, T. K. Shesagiri, M. Kumar, R. C. Hubli and A. K. Suri, Ind. Eng. Chem. Res., 2013, 52, 5400–5406 CrossRef CAS.
- A. Cieszynska and M. Wiśniewski, Hydrometallurgy, 2012, 113–114, 79–85 CrossRef CAS.
- J. Traeger, J. König, A. Städtke and H. J. Holdt, Hydrometallurgy, 2012, 127–128, 30–38 CrossRef CAS.
- A. Wołowicz and Z. Hubicki, Ind. Eng. Chem. Res., 2012, 51, 16688–16696 CrossRef.
- P. Chassary, T. Vincent, J. Sanchez Marcano, L. E. Macaskie and E. Guibal, Hydrometallurgy, 2005, 76, 131–147 CrossRef CAS.
- H. Sharififard, M. Soleinani and F. Z. Ashtiani, J. Taiwan Inst. Chem. Eng., 2012, 43, 696–703 CrossRef CAS.
- L. Liu, C. Li, C. Bao, Q. Jia, P. Xiao, X. Liu and Q. Zhang, Talanta, 2012, 93, 350–357 CrossRef CAS PubMed.
- V. Orr, L. Zhong, M. Moo-Young and C. P. Chou, Biotechnol. Adv., 2013, 31, 450–465 CrossRef CAS PubMed.
- E. Salehi, S. S. Madaeni and V. Vatanpour, J. Membr. Sci., 2012, 389, 334–342 CrossRef CAS.
- P. Daraei, S. S. Madaeni, E. Salehi, N. Ghaemi, H. S. Ghari, M. A. Khadivi and E. Rostami, J. Membr. Sci., 2013, 436, 97–108 CrossRef CAS.
- H. Deng, L. Gao, S. Zhang and J. Yuan, Ind. Eng. Chem. Res., 2012, 51, 14018–14025 CrossRef CAS.
- A. Mirmohseni, M. S. Seyed Dorraji, A. Figoli and F. Tasselli, Bioresour. Technol., 2012, 121, 212–220 CrossRef CAS PubMed.
- C. Xiong, L. Pi, X. Chen, L. Yang, C. Ma and X. Zheng, Carbohydr. Polym., 2013, 98, 1222–1228 CrossRef CAS PubMed.
- P. Baroni, R. S. Vieira, E. Meneghetti, M. G. da Silva and M. M. Beppu, J. Hazard. Mater., 2008, 152, 1155–1163 CrossRef CAS PubMed.
- D. S. Cocenza, M. A. Moraes, M. M. Beppu and L. F. Fraceto, Water, Air, Soil Pollut., 2012, 223, 3093–3104 CrossRef CAS.
- A. Heidari, H. Younesi, Z. Mehraban and H. Heikkinen, Int. J. Biol. Macromol., 2013, 61, 251–263 CrossRef CAS PubMed.
- M. M. Beppu, R. S. Vieira, C. G. Aimoli and C. C. Santana, J. Membr. Sci., 2007, 301, 126–130 CrossRef CAS.
- M. Liu, Z. Jia, D. Jia and C. Zhou, Prog. Polym. Sci., 2014, 39, 1498–1525 CrossRef CAS.
- A. R. Nesica, S. J. Velickovic and D. G. Antonovic, J. Hazard. Mater., 2012, 209–210, 256–263 CrossRef PubMed.
- P. Monvisade and P. Siriphannon, Appl. Clay Sci., 2009, 42, 427–431 CrossRef CAS.
- A. Olad and F. F. Azhar, Ceram. Int., 2014, 40, 10061–10072 CrossRef CAS.
- H. Hu, J. H. Xin, H. Hu, A. Chan and L. He, Carbohydr. Polym., 2013, 91, 305–313 CrossRef CAS PubMed.
- F. A. Pereira, K. S. Sousa, G. R. Cavalcanti, M. G. Fonseca, A. G. de Souza and A. P. Alves, Int. J. Biol. Macromol., 2013, 61, 471–478 CrossRef CAS PubMed.
- Z. Zhu, C. Gao, Y. Wu, L. Sun, X. Huang, W. Ran and Q. Shen, Bioresour. Technol., 2013, 147, 378–386 CrossRef CAS PubMed.
- J. M. Li, X. G. Meng, C. W. Hu and J. Du, Bioresour. Technol., 2009, 100, 1168–1173 CrossRef CAS PubMed.
- S. Chatterjee, M. W. Lee and S. H. Woo, Bioresour. Technol., 2010, 101, 1800–1806 CrossRef CAS PubMed.
- X. Li, Y. Qi, Y. Li, Y. Zhang, X. He and Y. Wang, Bioresour. Technol., 2013, 142, 611–619 CrossRef CAS PubMed.
- C. Bertagnolli, A. Uhart, J. C. Dupin, M. G. da Silva, E. Guibal and J. Desbrieres, Bioresour. Technol., 2014, 164, 264–269 CrossRef CAS PubMed.
- L. Bai, H. Hu, W. Fu, J. Wan, X. Cheng, L. Zhuge, L. Xiong and Q. Chen, J. Hazard. Mater., 2011, 195, 261–275 CrossRef CAS PubMed.
- F. Peirano, T. Vincent, F. Quignard, M. Robitzer and E. Guibal, J. Membr. Sci., 2009, 329, 30–45 CrossRef CAS.
- L. Chen, Z. Gao and Y. Li, Catal. Today, 2015, 245, 122–128 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– functional groups in the CCTS-M membrane played prominent roles in the adsorption process. Our results revealed that the membrane could be a promising adsorbent for the recovery of palladium.
N– functional groups in the CCTS-M membrane played prominent roles in the adsorption process. Our results revealed that the membrane could be a promising adsorbent for the recovery of palladium.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the amide group CONH– and the band at 1587 cm−1 was related to the deformation vibration of the amine group. The strong absorption band at around 1030 cm−1 was characteristic of pyranose rings of chitosan which matched well with the reported results.21 Compared to the CTS membrane, the spectrum of the CCTS-M membrane showed a combination of the characteristic absorptions of chitosan and Na-MMT groups. The –OH and Si–O–Si groups of Na-MMT (3618 cm−1 and 1032 cm−1) were also observed in the spectra of CCTS-M membranes, but these bands shifted to lower wavenumbers (3610 cm−1 and 1027 cm−1) in CCTS-M membranes, which were induced by the hydrogen bonding interaction between Na-MMT and chitosan. The intensity of the band at 1027 cm−1 increased, which might be because the C–O–C group vibration of CTS overlapped with the Si–O–Si stretching vibration of Na-MMT.29 Moreover, the new peak at 1650 cm−1 (C
O of the amide group CONH– and the band at 1587 cm−1 was related to the deformation vibration of the amine group. The strong absorption band at around 1030 cm−1 was characteristic of pyranose rings of chitosan which matched well with the reported results.21 Compared to the CTS membrane, the spectrum of the CCTS-M membrane showed a combination of the characteristic absorptions of chitosan and Na-MMT groups. The –OH and Si–O–Si groups of Na-MMT (3618 cm−1 and 1032 cm−1) were also observed in the spectra of CCTS-M membranes, but these bands shifted to lower wavenumbers (3610 cm−1 and 1027 cm−1) in CCTS-M membranes, which were induced by the hydrogen bonding interaction between Na-MMT and chitosan. The intensity of the band at 1027 cm−1 increased, which might be because the C–O–C group vibration of CTS overlapped with the Si–O–Si stretching vibration of Na-MMT.29 Moreover, the new peak at 1650 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and the shifting of peak (–NH2) by about 7 cm−1 can be attributed to the crosslinking reaction between glutaraldehyde and chitosan. In addition, the absorptions centered at about 3362 cm−1 and 3294 cm−1 reflected the amine groups in CCTS-M membrane. These FTIR results were comparable to the reports of glutaraldehyde-crosslinked chitosan,22,28 and indicated the formation of CCTS-M membrane. Significant changes of CCTS-M-Pd membrane were observed that the band at about 3294 cm−1 was related to –NH2 in unloaded membrane, and changed to 3223 cm−1 after adsorption of Pd(II) (Fig. 1b). The peak of Si–O–Si stretching vibration also shifted from 1027 cm−1 to 1038 cm−1. In addition, the –NH2 deformation vibration at 1593 cm−1 and the C
N) and the shifting of peak (–NH2) by about 7 cm−1 can be attributed to the crosslinking reaction between glutaraldehyde and chitosan. In addition, the absorptions centered at about 3362 cm−1 and 3294 cm−1 reflected the amine groups in CCTS-M membrane. These FTIR results were comparable to the reports of glutaraldehyde-crosslinked chitosan,22,28 and indicated the formation of CCTS-M membrane. Significant changes of CCTS-M-Pd membrane were observed that the band at about 3294 cm−1 was related to –NH2 in unloaded membrane, and changed to 3223 cm−1 after adsorption of Pd(II) (Fig. 1b). The peak of Si–O–Si stretching vibration also shifted from 1027 cm−1 to 1038 cm−1. In addition, the –NH2 deformation vibration at 1593 cm−1 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– stretching vibration at 1650 cm−1 were also observed to shift to 1577 cm−1 and 1623 cm−1 respectively, suggesting that a strong metal–ligand bond formed between Pd(II) and chelating groups involving the –NH2 and C
N– stretching vibration at 1650 cm−1 were also observed to shift to 1577 cm−1 and 1623 cm−1 respectively, suggesting that a strong metal–ligand bond formed between Pd(II) and chelating groups involving the –NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– groups in the CCTS-M membrane.
N– groups in the CCTS-M membrane.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– groups. At lower pH, the CCTS-M membrane was positively charged due to protonation of the amino groups, while the Pd(II) existed mostly as an anionic complex, viz. PdCl42− in hydrochloric acid solution, leading to an electrostatic attraction between them. The large excess of chloride anions and the strong competition between anions (Cl−) and PdCl42− restricted metal adsorption. However, with the increase of pH from 1 to 2, this competition decreased due to decrease in the amount of Cl−1 from HCl and the adsorption of metal increased.11 The maximum adsorption of Pd(II) on the CCTS-M membrane was 126 mg g−1 occurred at pH 2. Then the adsorption capacity decreased to 56 mg g−1 with further increased in the pH to 5, which may be attributed to the formation of hydrolyzed chloropalladium complex, to make the Pd(II) ions unavailable in the adsorption process. Additionally, the H+ concentration is insufficient for chitosan –NH2 to form the NH3+ to capture Pd(II) at high pH values.4 A much higher pH was not tested because of the possibility of Pd precipitation as Pd(OH)2. Therefore, pH 2 was chosen as the optimal pH condition in the following experiments.
N– groups. At lower pH, the CCTS-M membrane was positively charged due to protonation of the amino groups, while the Pd(II) existed mostly as an anionic complex, viz. PdCl42− in hydrochloric acid solution, leading to an electrostatic attraction between them. The large excess of chloride anions and the strong competition between anions (Cl−) and PdCl42− restricted metal adsorption. However, with the increase of pH from 1 to 2, this competition decreased due to decrease in the amount of Cl−1 from HCl and the adsorption of metal increased.11 The maximum adsorption of Pd(II) on the CCTS-M membrane was 126 mg g−1 occurred at pH 2. Then the adsorption capacity decreased to 56 mg g−1 with further increased in the pH to 5, which may be attributed to the formation of hydrolyzed chloropalladium complex, to make the Pd(II) ions unavailable in the adsorption process. Additionally, the H+ concentration is insufficient for chitosan –NH2 to form the NH3+ to capture Pd(II) at high pH values.4 A much higher pH was not tested because of the possibility of Pd precipitation as Pd(OH)2. Therefore, pH 2 was chosen as the optimal pH condition in the following experiments.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t
qe − K1t




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus ln
qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce.
Ce.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–. The adsorption capacity of the CCTS-M membrane adsorbent was found to be 196.07 mg g−1 according to the fitted parameter of the Langmuir equation. Moreover, the RL value of 0.58, which was between 0 and 1, confirmed that the conditions were favorable for the adsorption of Pd(II) onto the CCTS-M membrane.13
N–. The adsorption capacity of the CCTS-M membrane adsorbent was found to be 196.07 mg g−1 according to the fitted parameter of the Langmuir equation. Moreover, the RL value of 0.58, which was between 0 and 1, confirmed that the conditions were favorable for the adsorption of Pd(II) onto the CCTS-M membrane.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL
KL

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Si–O bonds, respectively.33 There were no obvious changes in the O 1s binding energies after Pd(II) adsorption (Fig. 6b and c), indicating that there were no or little chelating interactions between the groups containing oxygen and Pd(II).34 In the case of N 1s binding energy in high resolution XPS analysis, several peaks have been identified and their variations could be correlated with the changes in the chemistry of membrane after Pd(II) adsorption. The N 1s spectrum of CCTS-M showed three individual component peaks at 401.59 eV for –NH3+, 399.56 eV for –NH2 and 399.11 eV for –N
O and Si–O bonds, respectively.33 There were no obvious changes in the O 1s binding energies after Pd(II) adsorption (Fig. 6b and c), indicating that there were no or little chelating interactions between the groups containing oxygen and Pd(II).34 In the case of N 1s binding energy in high resolution XPS analysis, several peaks have been identified and their variations could be correlated with the changes in the chemistry of membrane after Pd(II) adsorption. The N 1s spectrum of CCTS-M showed three individual component peaks at 401.59 eV for –NH3+, 399.56 eV for –NH2 and 399.11 eV for –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, respectively (Fig. 6d). After adsorption, the N 1s binding energies of –NH2 and –N
C, respectively (Fig. 6d). After adsorption, the N 1s binding energies of –NH2 and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in CCTS-M-Pd were higher than that in CCTS-M, and shifted to 400.37 and 399.29 eV, respectively (Fig. 6e), due to donation of electrons from the N atoms of –NH2 and –N
C bonds in CCTS-M-Pd were higher than that in CCTS-M, and shifted to 400.37 and 399.29 eV, respectively (Fig. 6e), due to donation of electrons from the N atoms of –NH2 and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups to Pd(II), thereby indicating chelating interactions between these groups and Pd(II).35 As a consequence, the electron cloud density of the nitrogen atom was reduced, resulting in a higher observed binding energy peak. In addition, the peak at 401.73 eV assigned to –NH3+ increased significantly after Pd(II) adsorption, as seen in the Fig. 6e, possibly due to the formation of NH3+–(PdCl4)2−. The changes in the binding energies of –NH3+, –NH2 and –N
C groups to Pd(II), thereby indicating chelating interactions between these groups and Pd(II).35 As a consequence, the electron cloud density of the nitrogen atom was reduced, resulting in a higher observed binding energy peak. In addition, the peak at 401.73 eV assigned to –NH3+ increased significantly after Pd(II) adsorption, as seen in the Fig. 6e, possibly due to the formation of NH3+–(PdCl4)2−. The changes in the binding energies of –NH3+, –NH2 and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C indicated that N 1s was involved in the adsorption of Pd(II).36 These results accounted for the formation of CCTS-M–N⋯Pd complexes in the reactions of eqn (11)–(13). The high resolution Pd 3d spectrum of CCTS-M-Pd membrane was shown in Fig. 6f. The sample exhibited a Pd 3d5/2 peak belonging to the characteristic of divalent Pd at around 338 eV.36,37 It should be noted that the Pd 3d5/2 of CCTS-M-Pd exhibited two peaks at about 338.01 eV and 336.42 eV, indicating two different Pd environments: the peak at 336.42 eV was characteristic of the complexation formed between –N
C indicated that N 1s was involved in the adsorption of Pd(II).36 These results accounted for the formation of CCTS-M–N⋯Pd complexes in the reactions of eqn (11)–(13). The high resolution Pd 3d spectrum of CCTS-M-Pd membrane was shown in Fig. 6f. The sample exhibited a Pd 3d5/2 peak belonging to the characteristic of divalent Pd at around 338 eV.36,37 It should be noted that the Pd 3d5/2 of CCTS-M-Pd exhibited two peaks at about 338.01 eV and 336.42 eV, indicating two different Pd environments: the peak at 336.42 eV was characteristic of the complexation formed between –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/–NH2 groups and Pd(II); another peak at 338.01 eV corresponded to the Pd 3d5/2 electron binding energies of Pd(II).35 These can be ascribed to two complex ions for Pd in the adsorption process. Therefore, the possible adsorption mechanism can be described by eqn (10)–(13) and Fig. 7.
C/–NH2 groups and Pd(II); another peak at 338.01 eV corresponded to the Pd 3d5/2 electron binding energies of Pd(II).35 These can be ascribed to two complex ions for Pd in the adsorption process. Therefore, the possible adsorption mechanism can be described by eqn (10)–(13) and Fig. 7.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N + PdCl42− ↔ PdCl2(CCTS-M
N + PdCl42− ↔ PdCl2(CCTS-M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2 + 2Cl−
N)2 + 2Cl−
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– functional groups in CCTS-M membrane were confirmed to be involved in the Pd(II) adsorption process by XPS and FTIR techniques. These results are highly promising and suggest the CCTS-M membrane can be applied for recovery of palladium.
N– functional groups in CCTS-M membrane were confirmed to be involved in the Pd(II) adsorption process by XPS and FTIR techniques. These results are highly promising and suggest the CCTS-M membrane can be applied for recovery of palladium.







