In situ growth of biocidal AgCl crystals in the top layer of asymmetric polytriazole membranes†
Abstract
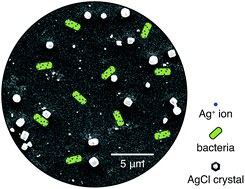
Scalable fabrication strategies to concentrate biocidal materials in only the surface of membranes are highly desirable. In this letter, tight-UF polytriazole membranes with a high concentration of biocide silver chloride (AgCl) crystals dispersed in only their top layer are presented. They were made following a simple dual-bath process that is compatible with current commercial membrane casting facilities. These membranes can achieve a 150-fold increase in their antimicrobial character compared to their silver-free counterpart. Moreover, fine-tuning of their properties is straightforward. A change in the silver concentration in one of the baths is sufficient to tune the permeance, molecular weight cut-off (MWCO) and silver loading of the final membrane.

 Please wait while we load your content...
Please wait while we load your content...