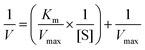Papain/Zn3(PO4)2 hybrid nanoflower: preparation, characterization and its enhanced catalytic activity as an immobilized enzyme†
Baoliang Zhang,
Peitao Li,
Hepeng Zhang*,
Lili Fan,
Hai Wang,
Xiangjie Li,
Lei Tian,
Nisar Ali,
Zafar Ali and
Qiuyu Zhang*
Key Laboratory of Applied Physics and Chemistry in Space, Ministry of Education, Department of Applied Chemistry, School of Science, Northwestern Polytechnical University, Youyi Road 127#, Xi'an, 710072, China. E-mail: zhanghepeng@nwpu.edu.cn; qyzhang@nwpu.edu.cn; Fax: +86-029-88431653; Tel: +86-029-88431675
First published on 28th April 2016
Abstract
Flower-like papain/Zn3(PO4)2 hybrid materials are synthesized via a facile, rapid and low-cost method in this study. The growth process of the nanoflowers has been studied in detail and a four-step formation mechanism, including coordination, precipitation, self-assembly and size growth, has been clarified. The concentration of papain mainly affects the morphology of the products by regulating the assembly and crystal growth. The enzyme activity of papain/Zn3(PO4)2 hybrid nanoflowers, a novel immobilized enzyme, was calculated by monitoring the hydrolysis reaction of casein. The results show that the catalytic properties of papain immobilized on hybrid nanoflowers are enhanced compared with that of free papain. The as-prepared hybrid nanoflowers exhibited excellent reusability, high thermo stability and long storage life. The results indicate that the well-designed materials have great potential in industrial applications.
1. Introduction
Papain (EC3.4.22.2), including 212 amino acid residues, is extracted from the latex of an unripe fruit named Carica papaya. This protease belongs to cysteine endopeptidase and can be used for hydrolysis of protein and terminal carboxy groups of arginine or lysine from polypeptide.1 Papain, which can be obtained easily in industry, is widely used in the field of food processing,2,3 catalysis,4,5 painting,6 bacteriostasis7 and detection8 because of its high enzyme activity, thermal and pH stability.In the past decade, the immobilization of biological molecules has evolved into a common technique to extend the catalytic activity.9–11 Enzyme immobilization is of great importance as it can increase the stability, catalytic activity and reusability of enzyme.12,13 For papain immobilization, various carriers have been designed and synthesized, including sepharose,14 polymer nanofibers,15 magnetic composite nanoparticles16 and modified mesoporous silica.17,18 The most frequently used method for immobilizing enzymes on the surface of the abovementioned carriers is covalent binding.15–18 However, the loading capacity of the enzyme is limited by the quantity of surface functional groups and the specific area of the carriers. As demonstrated by A. Bernkop-Schnürch et al.,19 the ionic gelation technique increases the loading quantity for papain immobilization on polyacrylic acid. Nevertheless, similar to covalent binding for enzyme immobilization, the enzyme activity was affected by the chemical process.
Based on the novel synthetic strategy of protein-inorganic hybrid nanoflowers that have been proposed,20 various enzymes have been chosen as the organic components.21–27 Studies of enzyme-inorganic hybrid nanoflowers suggest that the activity of enzyme–metal phosphate is enhanced.20,24,28 However, to the best of our knowledge, there has been no report yet about the use of papain for preparation of hybrid nanoflowers. In the present study, we employed the precipitation method to prepare papain/Zn3(PO4)2 hybrid nanoflowers. The objective of the current work is to apply the strategy to immobilize papain and to evaluate the activity of the as-synthesized hybrid nanoflowers. This type of designed material has shown to be a promising protocol to overcome the limitations of enzyme activity and loading quantity. Moreover, the advantages of the papain/Zn3(PO4)2 hybrid nanoflower can be displayed on the following aspects: (i) compared with the copper ion and the calcium ion, the zinc ion exhibits a rapid reaction rate with a phosphate radical29,30 and therefore the simple and rapid method can make industrial production easy to realize; (ii) the zinc ion has no destructive action on proteins31,32 and the immobilization method belongs to a physical process and thus the enzyme activity can be maintained; and (iii) the hybrid nanoflower shows high specific area and large pore size, which are favorable for mass transfer and contact reaction.
2. Experimental section
2.1 Materials
Papain from Carica papaya, tyrosine and casein were purchased from J&K Scientific Ltd. Zinc acetate, trichloroacetic acid (TCA), ethylenediaminetetraacetic acid (EDTA), KCl, NaCl, Na2HPO4, KH2PO4 and Na2CO3 were purchased from Sinopharm Chemical Reagent Co. Ltd. All the abovementioned chemical reagents were of pure analytical grade. Water used throughout the study was ultrapure produced by an apparatus for pharmaceutical purified water (Aquapro Co. Ltd.).PBS buffer was prepared as follows: 4.00 g of NaCl, 0.10 g of KCl, 0.72 g of Na2HPO4 and 0.12 g of KH2PO4 were dissolved in deionized water. The solution was collected in a 500 mL volumetric flask and cooled to room temperature. After that the solution was diluted with deionized water to 500 mL.
2.2 Preparation of papain/Zn3(PO4)2 hybrid nanoflowers
Papain/Zn3(PO4)2 hybrid nanoflowers were synthesized by the precipitation method. Typically, 0.0560 g of papain was dissolved in 70 mL of PBS buffer. The solution was added into a 150 mL flask. Under mechanical stirring, 5.6 mL of zinc acetate solution with the concentration of 0.05 g mL−1 was added into the flask. After being stirred for 3 h, papain/Zn3(PO4)2 hybrid nanoflowers were separated by centrifugation. The products were dried by vacuum freeze-drying.The effects of reaction temperature, additional amount of papain and agitation condition on the morphology of the hybrid nanoflowers and loading amount of papain were investigated. The specific experimental parameters are shown in Table S1.†
2.3 Determination of enzyme activity
Protease activity was determined at 37 °C in PBS buffer (pH 7.2) using casein as the substrate by the method of Ahmad Homaei.14 1 mL of free papain solution was added into a tube and the equilibrium temperature was 37 °C. 5 mL of casein was then added and the reaction mixture incubated at 37 °C for 10 min while continuously oscillating. The concentration of casein was 5 g L−1. The reaction was stopped by the addition of 5 mL of 50 g L−1 TCA solution. The mixture was incubated at 37 °C for 40 min. After filtration, the absorbance of the filtrate was measured at 275 nm. The same determination process was adopted for the hybrid nanoflowers. 0.1 g mL−1 of hybrid nanoflowers dispersion liquid was used instead of free papain solution. For the reference sample, the feeding order of TCA and casein was changed.2.4 Effect of pH and temperature on free and immobilized papain activity
The effect of temperature on the enzymatic activities of free papain and papain/Zn3(PO4)2 hybrid nanoflowers was measured in the temperature range of 25–90 °C. The effect of pH on the enzymatic activities was also assayed in the PBS buffers of pH ranging from 6.4 to 10.2. The standard enzyme activity assay procedure used was mentioned in 2.3.2.5 Determination of kinetic parameters for free and immobilized papain
The kinetic parameters of the Michaelis–Menten equation (Km and Vmax) for both free and immobilized papain were determined by measuring the initial rates of the reaction and varying casein concentration from 0.25 g mL−1 to 2.00 g mL−1 in PBS buffer pH = 7.4 at 37 °C. The kinetic parameters were evaluated by plotting the data using the Lineweaver–Burk and Eadie–Hofstee method. The Lineweaver–Burk equation can be written as follows:where [S] is the concentration of substrate and V and Vmax represent the initial and maximum rate of reactions, respectively. Km is the Michaelis–Menten constant (the substrate concentration when the rate is half of Vmax).33,34
2.6 Stabilities
The thermal stability of free papain and papain/Zn3(PO4)2 hybrid nanoflowers was studied by determining the residual activities of the enzymes after incubation in PBS buffer at 55 °C for 390 min with continuous shaking. A sample was taken at regular time intervals and assayed for enzyme activity. The relative activity (%) was the ratio between the activity of every sample and the maximum activity of the sample. The storage stability of free papain and papain/Zn3(PO4)2 hybrid nanoflowers was studied at 4 °C for 36 days and the residual activities of the papain were measured with a time interval of 3 days. The reusability of free papain and papain/Zn3(PO4)2 hybrid nanoflowers was determined at 37 °C. The same enzyme activity assay was used in ten repeated cycles. For every repetitive cycle, the hybrid nanoflowers were removed from the reaction medium and washed with PBS buffer three times.2.7 Characterization
The average diameter and particle size distribution of the papain/Zn3(PO4)2 hybrid nanoflowers were determined using an LS13320 Laser Particle Size Analyzer (Beckman Coulter). The testing system was deionized water. Fourier transform infrared (FTIR) spectra were acquired on a TENSOR27 FTIR spectrometer (Bruker). The morphology of the nanoflowers was observed using a Scanning Electron Microscope (SEM, JEOL JSM-6700F). Elemental composition of the hybrid microspheres was analyzed on an elemental analyzer (Vario EL III, Elementar Analysensysteme GmbH) with CHN mode. Specific surface areas and pore size distribution were computed from the results of N2 physisorption (Tristar3020, Micromeritics) using the BET (Brunauer–Emmet–Teller). Powder X-ray diffraction (XRD) patterns were recorded on a Shimadzu XRD-6000 diffractometer using Cu Kα radiation. The enzyme activity of the samples was analyzed using a UV-vis spectrophotometer (BlueStar, LabTech).3. Results and discussion
3.1 Composition of papain/Zn3(PO4)2 hybrid nanoflowers
The composition of the product was characterized by X-ray powder diffraction (XRD) and Fourier transform infrared (FTIR). The XRD patterns of papain, Zn3(PO4)2 nanoparticles and typical papain/Zn3(PO4)2 hybrid nanoflowers are shown in Fig. 1A. All the diffraction peaks in the XRD spectrum of Zn3(PO4)2 nanoparticles are in good agreement with Zn3(PO4)2·4H2O, which can be indexed by JCPDS (card no. 33-1474). The spectrum of the papain/Zn3(PO4)2 hybrid nanoflowers contained all the peaks belonging to Zn3(PO4)2 nanoparticles, indicating that inorganic composition of the as-prepared hybrid nanoflowers was zinc phosphate tetrahydrate. In addition, the peaks at 12.56°, 19.46° and 37.62° attributed to papain were also observed in the spectrum of the papain/Zn3(PO4)2 hybrid nanoflowers. The spectra of the samples from 400 cm−1 to 4000 cm−1 are shown in Fig. 1B. Typical absorption peaks of pure papain occurred at 1340–1430 and 1542 cm−1 for –CONH and 2800–3000 cm−1 for CH2 and CH3. The strong characteristic absorption peaks at 1014, 950 and 634 cm−1, which were observed in the spectrum of Zn3(PO4)2 nanoparticles, were attributed to P–O vibrations.35 It can be seen that the characteristic absorption peaks of pure papain and Zn3(PO4)2 are maintained in the spectrum of papain/Zn3(PO4)2 hybrid nanoflowers. These results demonstrated that the nanoflowers were formed by Zn3(PO4)2·4H2O and papain.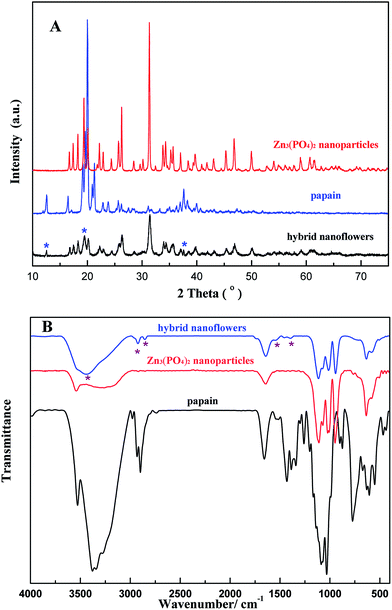 | ||
| Fig. 1 X-ray diffraction patterns (A) and FTIR spectra (B) of pure papain, Zn3(PO4)2 nanoparticles and papain/Zn3(PO4)2 hybrid nanoflowers. | ||
3.2 Controlled synthesis of papain/Zn3(PO4)2 hybrid nanoflowers
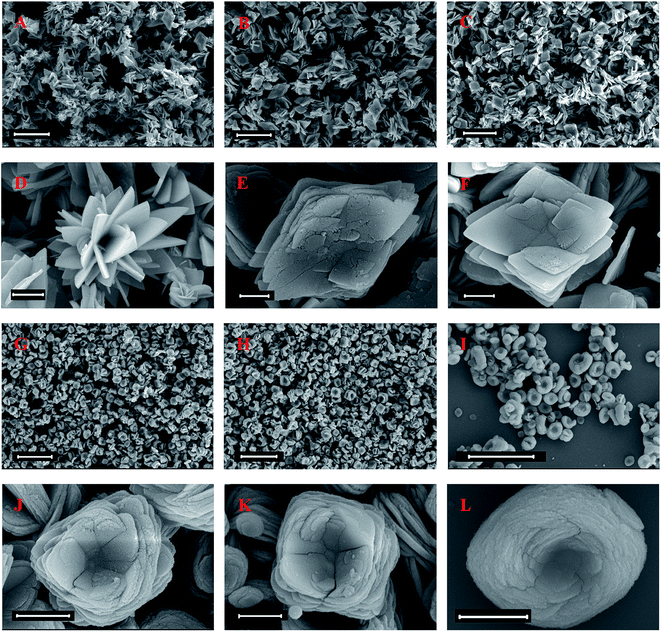
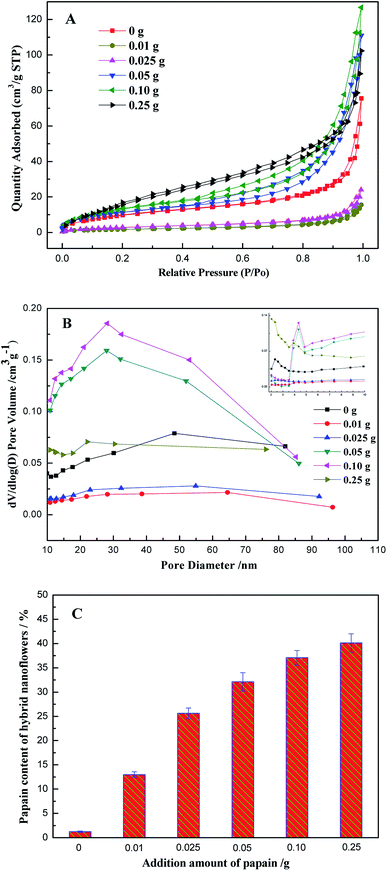
The enzyme concentration was confirmed to have a crucial effect on the morphology of the papain/Zn3(PO4)2 hybrid nanoflowers.20 Thus, the effect of an additional amount of papain was estimated, and the result is shown in Fig. 2. Compared with pure Zn3(PO4)2 nanoparticles (Fig. 2A and D), the morphologies of the products prepared in the presence of papain changed distinctly. When the amount of papain was lower than 0.025 g, the hybrid nanoflowers displayed rhombic shapes with a multi-layered stacking structure, as shown in Fig. 2B, C, E and F. With the increase of additional amounts of papain, the morphology of the products changed from “rhombus” to “square”, and the layers increased (Fig. 2D, G, H and J). When the amount of papain was increased up to 0.25 g, oblate spheroid hybrid nanomaterials, which looked like blooming flowers, were obtained as the dominant product (Fig. 2I and L). The flower shape had no significant change with the continuous increase of papain; however, the particle size slightly increased (see the ESI, Fig. S1†). From the abovementioned results, it can be concluded that the presence of papain affected the assembly and crystal growth of papain/Zn3(PO4)2 hybrid materials.Fig. 3A and B are N2 adsorption–desorption isotherms and pore size distributions of the products. From the BET curves, it can be seen that the specific area of the papain/Zn3(PO4)2 hybrid nanoflowers, prepared using less than 0.025 g of papain, was lower than that of the pure Zn3(PO4)2 nanoparticles (38.68 m2 g−1). The specific area of the hybrid nanoflowers increased with increasing amounts of papain, which was in good agreement with the results determined by SEM showing increased layers. When the dosages of papain were 0.05 g, 0.10 g and 0.25 g, the specific areas of the papain/Zn3(PO4)2 hybrid nanoflowers were 43.23, 51.24 and 70.06 m2 g−1, respectively. It was found that the adsorption–desorption isotherms belonged to the type IV isotherm (isotherm with hysteresis loop) according to IUPAC classification, and the hysteresis loops were the H3 type. Such a hysteresis loop type indicated that the nanoflowers contained slit-type pores. This might be attributed to close packing of the “petals” of the hybrid nanoflowers. Mesopores and macropores were observed in the hybrid nanoflowers from the curves of pore size distributions (Fig. 3B). The number of mesopores increased with increasing dosage of papain. The large pore channel exhibited an advantage for mass transfer.36 The relationship between the papain content of papain/Zn3(PO4)2 hybrid nanoflowers and the additional amount of papain was investigated, and the result is shown in Fig. 3C. The papain content increased drastically at first then a slow but gradual increase was observed.
The influence of the dosage of zinc ion on the morphology of the papain/Zn3(PO4)2 hybrid nanoflowers was also studied. As shown in Fig. 4, 0.25 g and 0.005 g of papain were chosen for investigation. The products prepared under 0.25 g of papain exhibited oblate spheroid shapes (Fig. 4A–D). The layer number of nanoplate increased with increasing amounts of zinc ion. The ample dosage of papain limited the morphology and provided more raw materials for the growth of hybrid nanoflowers. It is evident that the morphology of hybrid nanoflowers synthesized by 0.0050 g of papain significantly changed with an increase in zinc ion (Fig. 4E–H). Compared with the hybrid nanoflowers prepared using 0.25 g of papain, in addition to the difference in morphologies, the particle size of the hybrid nanoflowers prepared under 0.0050 g of papain also increased evidently. The change of particle size may be ascribed to the following two reasons: first, papain has the ability to limit the crystal form, decreasing its dosage, and the control ability of the crystal form was relatively weakened and the size of the nanoplates increased; second, papain played a role as a binder in the assembly process of zinc phosphate, and the zinc phosphate nanoplates grew independently and rapidly when the dosage of papain was lower. According to the results, it is reasonable to assume that firstly the papain/Zn2+ complex was formed (zinc acetate was 0.14 g, Fig. 4E), followed by the formation of nanoplates and then the assembly of nanoplates. When the dosage of zinc acetate was more than 0.14 g (Fig. 4F–H), zinc phosphate nanocrystals were formed by in situ nucleation on the complex, which can be regarded as a core. Then, the assembly of zinc phosphate nanoplates centered on the core.
3.3 Formation mechanism of papain/Zn3(PO4)2 hybrid nanoflowers
To understand the formation mechanism of the papain/Zn3(PO4)2 hybrid nanoflowers, time-dependent experiments were performed, in which intermediate products were collected at different intervals. All the intermediate products were monitored by SEM and Elemental Analyzer, and the results are shown in Fig. 5 and 6. Nanoparticles and soft materials were obtained within 10 min (Fig. 5A). The nanoparticles were Zn3(PO4)2 and the soft materials were papain. Monolayer nanoplates triangular or quadrangular in shape were observed at 15 min (Fig. 5B). The nanoplates started to assemble when the reaction time was 30 min. The precursors of hybrid nanoflowers, which were formed with multilayer nanoplates, were prepared and are shown in Fig. 5C. With the increase of reaction time, the extent of self-assembly became greater. The layer number of papain/Zn3(PO4)2 hybrid nanoflowers continually increased (Fig. 5D–F). It can be seen from Fig. 6 that the papain content decreased when the reaction time was prolonged. At the initial stage, the complexes and zinc phosphate nanocrystals were the main products. Therefore, the papain content was high. With the rapid formation of Zn3(PO4)2, the papain content decreased. It was inferred from this that the complexes and nanocrystals preferentially formed and then the continuous growth of zinc phosphate and self-assembly process appeared in succession.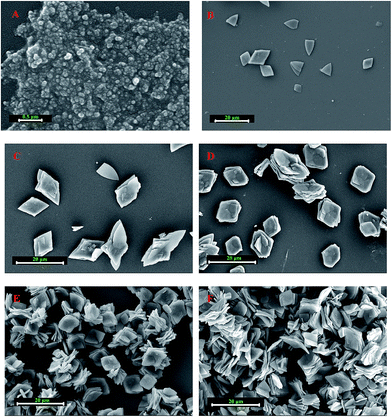 | ||
| Fig. 5 SEM images of papain/Zn3(PO4)2 hybrid nanoflowers formed during different periods: 10 min (A); 15 min (B); 30 min (C); 60 min (D); 120 min (E); 180 min (F). | ||
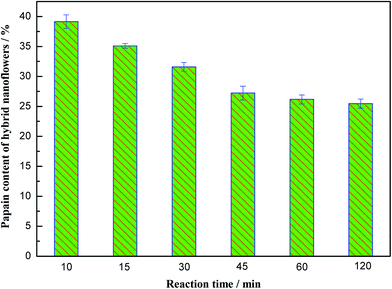 | ||
| Fig. 6 The reaction time dependence of papain content of papain/Zn3(PO4)2 hybrid nanoflowers (error bars represent ± standard deviations, n = 3). | ||
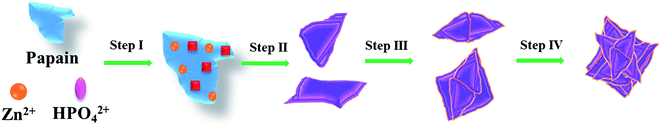
On the basis of the abovementioned results, a four-step mechanism for formation of papain/Zn3(PO4)2 hybrid nanoflowers was proposed, which is schematically illustrated in Fig. 7. Initially, the coordination of Zn2+ and papain was carried out and zinc phosphate nanocrystals were prepared by in situ nucleation of Zn2+ and phosphate simultaneously. In a second stage, the Zn3(PO4)2 crystal nucleus continuously grew and the monolayer nanoplates were obtained. These were the assembly units which can be seen as “petals”. While in the third stage, the nanoplates assembled to form the 3D structure, just as the morphology shows in Fig. 6C and D. However, the layers were relatively few. At last, the assembly process was carried out continuously. The plates arranged compactly and the interleaved pores were constructed. Then, the completely hybrid nanoflowers with larger size were acquired.
3.4 Application properties of papain/Zn3(PO4)2 hybrid nanoflowers
The effect of temperature on the hydrolysis activity of free and as-synthesized papain/Zn3(PO4)2 hybrid nanoflowers for casein was determined, and the results are shown in Fig. 8A. It can be seen that the optimum temperatures for free papain and the hybrid nanoflowers were determined to be 55 °C and 70 °C, respectively. Moreover, the hybrid nanoflowers were most active in the range of 45–80 °C, which was wider than that of free papain (50–70 °C). The abovementioned advantages of immobilized papain may be caused by decreasing the amount of papain that comes in contact with each other and increasing of conformational rigidity.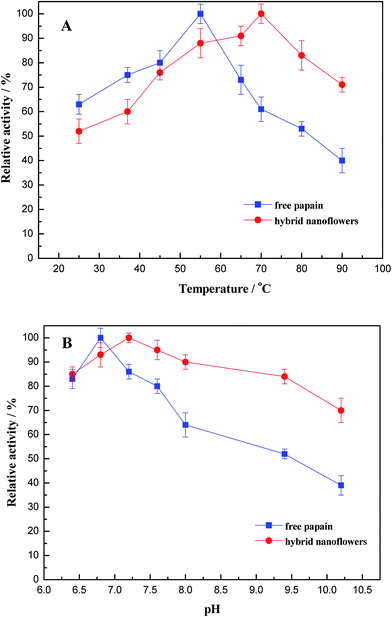 | ||
| Fig. 8 The effect of temperature (A) and pH (B) on the activity of free papain and papain/Zn3(PO4)2 hybrid nanoflowers (error bars represent ± standard deviations, n = 3). | ||
The effect of pH on the enzyme activity of the resulting papain/Zn3(PO4)2 hybrid nanoflowers and free papain was also examined in the present study, as shown in Fig. 8B. The suitable pH range for hybrid nanoflowers was 6.5–9.5. The relative activity was more than 80%. However, the free papain was most active in the range of 6.5–8.0, and the enzyme activity decreased rapidly with the increase of pH value. The enhancement of the catalytic property of hybrid nanoflowers can most probably be attributed to the immobilization of papain, which prevents not only the conformational change but also the denaturation of papain.
The protein hydrolysis kinetics of free and immobilized papain using casein as a substrate were examined, and the result is shown in Fig. 9. The calculated data demonstrated that the kinetics constants Km and Vmax of free papain were 0.245 g mL−1 and 3.178 mg mL−1 min−1, respectively. For immobilized papain, Km and Vmax values were 0.252 g mL−1 and 2.564 mg mL−1 min−1. The slight increase in the Km value of the immobilized papain demonstrated that the concentration of substrate casein required for immobilized papain is greater than that required for free papain under identical conditions.34 This might be due to the decrease in affinity of papain for casein probably caused by diffusional limitation of substrate to the active site of the enzyme.33
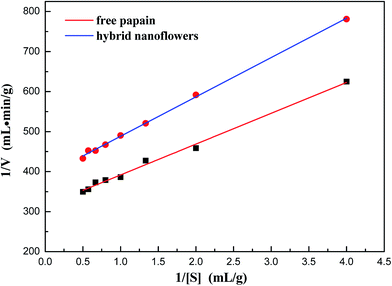 | ||
| Fig. 9 Lineweaver–Burk plot for the maximum hydrolysis rate (Vmax) of free papain and papain/Zn3(PO4)2 hybrid nanoflowers. | ||
In order to decrease the cost of enzymes used for industrial purposes, high thermo stability and long storage life are required. These are also the advantages of the immobilized enzyme. The thermo and storage stabilities of papain/Zn3(PO4)2 hybrid nanoflowers were evaluated. The immobilized papain on the hybrid nanoflower was found to be more thermostable than free papain (Fig. 10A). The hybrid nanoflower and free papain retained 77.2% and 41.9% of activity, respectively, at 55 °C after 390 min. It can be seen from Fig. 10B that the free enzyme lost 62.5% of its initial activity over a 36 day period, whereas the papain/Zn3(PO4)2 hybrid nanoflowers lost only 17.7% of its initial activity under the same storage conditions.
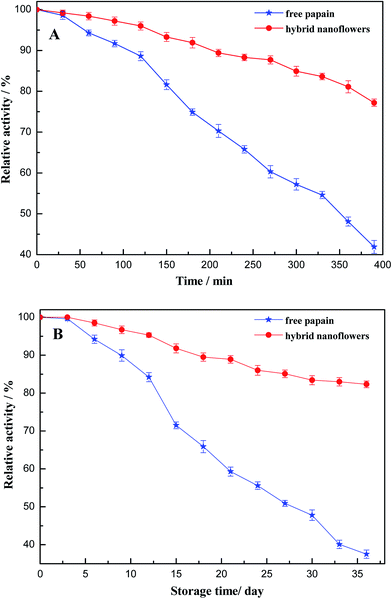 | ||
| Fig. 10 Effect of thermal stability (A) and storage stability (B) of free papain and papain/Zn3(PO4)2 hybrid nanoflowers (error bars represent ± standard deviations, n = 3). | ||
The reusability of papain/Zn3(PO4)2 hybrid nanoflowers was investigated in this study. As shown in Fig. 11, the enzyme activity of the recycled hybrid nanoflowers decreased slowly as the cycle number increased. The hybrid nanoflowers retained 88.8% of its initial activity after ten successive cycles of reuse. A comparison of the performance of immobilized papain on different support materials is given in Table 1. On comparing the as-prepared papain/Zn3(PO4)2 hybrid nanoflowers with previously reported carriers, it could be found that the designed immobilized papain exhibited excellent storage stability, reusability and operational stability.
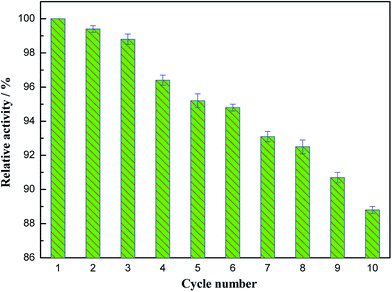 | ||
| Fig. 11 Reusability of papain/Zn3(PO4)2 hybrid nanoflowers (error bars represent ± standard deviations, n = 3). | ||
| Immobilization support | Adsorption capacity (mg g−1) | Storage stabilitya | Reuse stability | References | ||
|---|---|---|---|---|---|---|
| Residual activityb | Storage time (days) | Residual activityb | Cycle numbers | |||
| a The storage stability of the immobilized papain was studied at 4 °C.b Residual activity of the immobilized papain with the initial activity of the immobilized papain set as 100%.c The data were read from the curves which are shown in the references. | ||||||
| Papain/Zn3(PO4)2 hybrid nanoflowers | 321.14 | 82.3% | 36 | 88.8% | 10 | This work |
| CNBr-activated sepharose | 36.5 | 80%c | 60 | 83% | 7 | 14 |
| Poly(vinyl alcohol) nanofibers | 130 | 40% | 14 | 12% | 6 | 15 |
| Silica-coated magnetic nanoparticles | 187.072 | 60%c | 30 | 75.15% | 10 | 16 |
| Cellulose nanocrystals/Fe3O4/Au | 186 | 95% | 35 | 80% | 12 | 37 |
| Papain/Cu3(PO4)2·3H2O hybrid nanoflowers | 391.5 | — | — | 28.9% | 5 | 38 |
| Biosilica matrix | — | 80 | 30 | 93%c | 7 | 39 |
| Poly(methacrylic acid)-grafted chitosan/clay | 34.47 | — | — | 43% | 10 | 40 |
4. Conclusion
The present study demonstrates a simple and rapid strategy to achieve papain/Zn3(PO4)2 hybrid nanoflowers. The resultant hybrid nanoflower formed by papain and zinc phosphate tetrahydrate is a type of immobilized enzyme that exhibits excellent bio-catalytic performance, not only because of the reusability and operational stability, but also because of the high thermo stability and long storage life. Therefore, the as-prepared hybrid materials have great potential in industrial applications.Acknowledgements
The authors are grateful for the financial support provided by the State Key Program of National Natural Science of China (Grant No. 51433008), the Natural Science Foundation of Shaanxi Province (Grant No. 2015JQ2055, 2015JM2050), and the Basic Research Fund of Northwestern Polytechnical University (Grant No. 3102014JCQ01094, 3102014ZD).References
- Y. He, S. M. S. bin Tuan Chik and F. C. Chong, J. Ind. Eng. Chem., 2014, 20, 4293 CrossRef CAS.
- E. Hatta, K. Matsumoto and Y. Honda, J. Cereal Sci., 2015, 61, 41 CrossRef CAS.
- Y. Li, J. Yu, I. Goktepe and M. Ahmedna, Food Chem., 2016, 196, 1338 CrossRef CAS PubMed.
- J. Mitsuhashi, T. Nakayama and A. Narai-Kanayama, Enzyme Microb. Technol., 2015, 75–76, 10 CrossRef CAS PubMed.
- P. Alpay and D. A. Uygun, J. Mol. Catal. B: Enzym., 2015, 111, 56 CrossRef CAS.
- R. S. Peres, E. Armelin, J. A. Moreno-Martínez, C. Alemán and C. A. Ferreira, Appl. Surf. Sci., 2015, 341, 75 CrossRef CAS.
- M. M. Dos Anjos, A. A. da Silva, I. C. de Pascoli, J. M. Mikcha, M. Machinski Jr, R. M. Peralta and B. A. de Abreu Filho, Int. J. Food Microbiol., 2016, 216, 121 CrossRef CAS PubMed.
- Y. Chen, Y. Wang, C. Wang, W. Li, H. Zhou, H. Jiao, Q. Lin and C. Yu, J. Colloid Interface Sci., 2013, 396, 63 CrossRef CAS PubMed.
- Y. Cao, L. Wen, F. Svec, T. Tan and Y. Lv, Chem. Eng. J., 2016, 286, 272 CrossRef CAS.
- E. Seymour, G. G. Daaboul, X. Zhang, S. M. Scherr, N. L. Unlu, J. H. Connor and M. S. Unlu, Anal. Chem., 2015, 87, 10505 CrossRef CAS PubMed.
- H. Nishida, T. Kajisa, Y. Miyazawa, Y. Tabuse, T. Yoda, H. Takeyama, H. Kambara and T. Sakata, Langmuir, 2015, 31, 732 CrossRef CAS PubMed.
- O. Barbosa, C. Ortiz, A. Berenguer-Murcia, R. Torres, R. C. Rodrigues and R. Fernandez-Lafuente, Biotechnol. Adv., 2015, 33, 435 CrossRef CAS PubMed.
- S. Sulaiman, M. N. Mokhtar, M. N. Naim, A. S. Baharuddin and A. Sulaiman, Appl. Biochem. Biotechnol., 2015, 175, 1817 CrossRef CAS PubMed.
- A. Homaei, Int. J. Biol. Macromol., 2015, 75, 373 CrossRef CAS PubMed.
- I. E. Moreno-Cortez, J. Romero-Garcia, V. Gonzalez-Gonzalez, D. I. Garcia-Gutierrez, M. A. Garza-Navarro and R. Cruz-Silva, Mater. Sci. Eng., C, 2015, 52, 306 CrossRef CAS PubMed.
- L. Mosafa, M. Moghadam and M. Shahedi, Chin. J. Catal., 2013, 34, 1897 CrossRef CAS.
- W. Bian, L.-L. Lou, B. Yan, C. Zhang, S. Wu and S. Liu, Microporous Mesoporous Mater., 2011, 143, 341 CrossRef CAS.
- W. Bian, B. Yan, N. Shi, F. Qiu, L.-L. Lou, B. Qi and S. Liu, Mater. Sci. Eng. C, 2012, 32, 364 CrossRef CAS.
- C. Muller, G. Perera, V. Konig and A. Bernkop-Schnürch, Eur. J. Pharm. Biopharm., 2014, 87, 125 CrossRef CAS PubMed.
- J. Ge, J. Lei and R. N. Zare, Nat. Nanotechnol., 2012, 7, 428 CrossRef CAS PubMed.
- L. B. Wang, Y. C. Wang, R. He, A. Zhuang, X. Wang, J. Zeng and J. G. Hou, J. Am. Chem. Soc., 2013, 135, 1272 CrossRef CAS PubMed.
- J. Sun, J. Ge, W. Liu, M. Lan, H. Zhang, P. Wang, Y. Wang and Z. Niu, Nanoscale, 2014, 6, 255 RSC.
- Z. Lin, Y. Xiao, Y. Yin, W. Hu, W. Liu and H. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 10775 CAS.
- Y. Yin, Y. Xiao, G. Lin, Q. Xiao, Z. Lin and Z. Cai, J. Mater. Chem. B, 2015, 3, 2295 RSC.
- Z. Zhang, Y. Zhang, R. Song, M. Wang, F. Yan, L. He, X. Feng, S. Fang, J. Zhao and H. Zhang, Sens. Actuators, B, 2015, 211, 310 CrossRef CAS.
- R. Wang, Y. Zhang, D. Lu, J. Ge, Z. Liu and R. N. Zare, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2013, 5, 320 CrossRef CAS PubMed.
- D. Liu, Z. Wang and X. Jiang, Nanoscale, 2011, 3, 1421 RSC.
- L. Fang, B. Zhang, W. Li, J. Zhang, K. Huang and Q. Zhang, Electrochim. Acta, 2014, 148, 164 CrossRef CAS.
- B. Zhang, P. Li, H. Zhang, X. Li, L. Tian, H. Wang, X. Chen, N. Ali, Z. Ali and Q. Zhang, Appl. Surf. Sci., 2016, 366, 328 CrossRef CAS.
- B. Zhang, P. Li, H. Zhang, H. Wang, X. Li, L. Tian, N. Ali, Z. Ali and Q. Zhang, Chem. Eng. J., 2016, 291, 287 CrossRef CAS.
- T. Kochanczyk, A. Drozd and A. Krezel, Metallomics: integrated biometal science, 2015, 7, 244 RSC.
- W. Maret, Metallomics, 2015, 7, 202 RSC.
- B. Sahoo, S. K. Sahu, D. Bhattacharya, D. Dhara and P. Pramanik, Colloids Surf., B, 2013, 101, 280 CrossRef CAS PubMed.
- L. Sun, H. Liang, Q. Yuan, T. Wang and H. Zhang, J. Chem. Technol. Biotechnol., 2012, 87, 1083 CrossRef CAS.
- Y. Yu, X. Fei, J. Tian, L. Xu, X. Wang and Y. Wang, Colloids Surf., B, 2015, 130, 299 CrossRef CAS PubMed.
- F. Gritti and G. Guiochon, J. Chromatogr. A, 2015, 1392, 10 CrossRef CAS PubMed.
- K. A. Mahmoud, E. Lam, S. Hrapovic and J. H. Luong, ACS Appl. Mater. Interfaces, 2013, 5, 4978 CAS.
- L. Liang, X. Fei, Y. Li, J. Tian, L. Xu, X. Wang and Y. Wang, RSC Adv., 2015, 5, 96997 RSC.
- L. Zhou, C. Wang, Y. Jiang and J. Gao, Chin. J. Chem. Eng., 2013, 21, 670 CrossRef CAS.
- A. U. Metin and E. Alver, Bioprocess Biosyst. Eng., 2016 DOI:10.1007/s00449-016-1590-0.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05308d |
| This journal is © The Royal Society of Chemistry 2016 |