Nitrogen-doped heterostructure carbon functionalized by electroactive organic molecules for asymmetric supercapacitors with high energy density†
Abstract
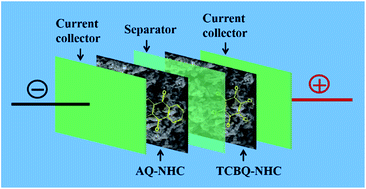
Chemical oxidation is employed to lengthwise unzip and transverse cut multi-walled carbon nanotubes (MWCNTs) to form heterostructure carbon nanotubes (HCNTs) that are residual tubes with randomly distributed graphene layers on the tube wall. Then, we coat polyaniline nanoparticles on HCNTs through in situ polymerization, in which the HCNTs are served as core and polyaniline is regarded as shell. The resultant core–shell structure is converted to a nitrogen-doped heterostructure carbon (NHC) through pyrolysis by following alkali activation. Subsequently, the NHC is used as conductive substrate to adsorb tetrachlorobenzoquinone (TCBQ) and anthraquinone (AQ) molecules via π–π stacking interaction to get the functionalized nitrogen-doped heterostructure carbon (TCBQ–NHC and AQ–NHC), respectively. As a result, multielectron reactions in positive and negative potential ranges are implanted in two electrodes, respectively. Electrochemical measurements show that the TCBQ–NHC and AQ–NHC electrodes achieve specific capacitances of 365 and 331 F g−1 at 1 A g−1 in potential windows of 0–1.0 and −0.4 to 0.6 V, respectively. Furthermore, the as-constructed AQ–NHC//TCBQ–NHC asymmetric supercapacitor (ASC) can deliver high energy density (20.3 W h kg−1) at the power density of 0.7 kW kg−1 with long cycle life (the capacitance remains 98% of the initial value after 5000 cycles).

 Please wait while we load your content...
Please wait while we load your content...