Magnetic Co0.5Zn0.5Fe2O4 nanoparticle-modified polymeric g-C3N4 sheets with enhanced photocatalytic performance for chloromycetin degradation†
Abstract
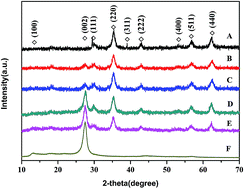
The visible-light and heterojunctional photocatalyst Co0.5Zn0.5Fe2O4/g-C3N4 (CN-CZF) was prepared for the first time in a hydrothermal route by adopting Co0.5Zn0.5Fe2O4 and g-C3N4 as monomer. This synthetic method is simple and mild. The activity of the photocatalyst was evaluated by measuring the rate of the degradation of chloromycetin under visible light irradiation. Among the prepared photocatalysts, CN-CZF4 exhibits the highest photocatalytic activity, the reaction rate constant of which is 2.5 times that of pure g-C3N4. The increased photocatalytic activity of CN-CZF composites can be attributed to the formation of a heterojunction between Co0.5Zn0.5Fe2O4 and g-C3N4, which suppresses the recombination of photoinduced electron–hole pairs. Meanwhile, the interface between g-C3N4 and Co0.5Zn0.5Fe2O4 was very important for the photocatalytic activity as determined by comparative tests. A possible photocatalytic mechanism of the CN-CZF composite is proposed.

 Please wait while we load your content...
Please wait while we load your content...