pH- and concentration-controlled self-assembly of spherical micelles with cavity, necklace and cylindrical micelles†
Rui Qiab and
Yong Jin*cd
aCenter of Polymer Science and Technology, Chengdu Institute of Organic Chemistry, Chinese Academy of Science, Chengdu 610041, China
bUniversity of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, China
cNational Engineering Laboratory for Clean Technology of Leather Manufacture, Sichuan University, Chengdu 610065, China
dKey Laboratory of Leather Chemistry and Engineering (Sichuan University), Ministry of Education, Chengdu 610065, China. E-mail: jinyong@cioc.ac.cn
First published on 4th May 2016
Abstract
A novel diblock copolymer with one block alternatively connected with hydrophobic motifs, hydrogen-bonding carbamates and pH-triggered carboxy groups was developed, which showed an exciting pH- and concentration-dependent self-assembly of spherical micelles with cavity, necklaces and cylindrical micelles.
The self-assembly of amphiphilic block copolymers has attracted great interest in recent decades, due to the creation of well-defined nanostructures with abundant morphologies and diverse functionalities.1–3 The properties and applications of the nanostructures are highly dependent on their sizes and shapes that are governed by the characters of the block copolymers and environmental conditions.4–7 Thus, the exploitation of the molecular structures and the environmental conditions is essential for the engineering of targeted assemblies. Incorporation of functional groups into the block copolymers could usually bring about opportunities to construct nanostructures with varied morphologies including spherical micelles,8 cylindrical micelles,9 vesicles,10 nanorods,11 nanorings,12,13 etc. Hydrogen-bonding groups14–20 and pH-triggered acid groups21–25 were both demonstrated to play key roles in the morphology-controlled self-assembly. Therefore, the combination of hydrogen-bonding groups and pH-triggered acid groups in the block copolymers would probably direct an exciting self-assembly. However, research focusing on the self-assembly of block copolymers with one of the blocks containing hydrogen-bonding groups and the other pH-triggered carboxy groups has been studied much less.
Herein, we developed a novel diblock copolymer with one block alternatively connected with hydrophobic motifs, hydrogen-bonding carbamates and pH-triggered carboxy groups, which showed an exciting pH- and concentration-dependent self-assembly of spherical micelles with cavity, necklaces and cylindrical micelles (Scheme 1).
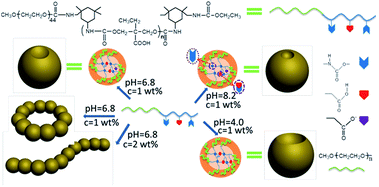 | ||
| Scheme 1 Schematic representations of possible supramolecular structures of the diblock copolymer assemblies. | ||
The synthesis of the diblock copolymer started with the preparation of the block alternatively connected with the isophorone diisocyanate (IPDI) and 2,2-dimethylolbutyric acid (DMBA) according to the procedures described previously.12 Then diblock copolymer was prepared by the reaction of the isocyanate and hydroxyl group of the poly(ethylene glycol) methyl ether (MPEG) (Scheme S1†). The resulting molecule was successfully characterized by 1H NMR spectroscopy (Fig. S1†), FT-IR (Fig. S2†) and GPC (Fig. S3†), respectively, and are shown to be in full agreement with the structure designed.
In aqueous solutions, the hydrophobic cyclohexyl groups and the hydrogen-bonding carbamates act as bridge bond to drive the self-assembly of the diblock copolymer into nanoparticles. Meanwhile, the pH-triggered carboxy groups play a key role in the morphology of the assembled nanoparticles, because of the pH-triggered carboxy groups could transform from hydrophobic electric neutrality to hydrophilic charged groups with the variation of the environment pH values.
In aqueous solutions, the diblock copolymer could solve in the form of single molecule at the pH value of 9.2, but the diblock copolymers were able to self-assemble into micelles at the pH values below 8.2, which was confirmed by the dynamic light scattering (DLS) measurements (Fig. S4†). The formation of the micelles was considerably governed by the variation of the hydrophilicity of the block copolymer. For the systems of pH at 9.2, the carboxy groups entirely ionized, resulting in that the diblock copolymer was single molecule in the solution. In contrast, in the systems with the pH values below 8.2, the diblock copolymer could self-assemble into micelles.
To investigate the effects of the pH values on the self-assembly behavior of the diblock copolymer, three different pH aqueous solutions with a fixed concentration at c = 1 wt% were studied in detail by the scan probe microscope (SPM) measurements. Fig. 1a shows that a series of spherical micelles with the sizes ranging from 50 to 90 nm were detected in the solution with the pH value of 8.2. The sizes of these spherical micelles observed by SPM were corresponding to the DLS result of Fig. S4b.† Notably, each micelle possesses a small cavity on the surface. For the system with pH of 6.8, the spherical micelles with cavities were still observed (Fig. 1b), which was also proved by the transmission electron microscopy (TEM) experiments (Fig. S5†). Fig. 1c shows the 3D morphological structures of the spherical micelles, which further confirm the existence of the cavity on the surface of each spherical nanoparticle. Comparing the difference of the spherical micelles formed in the systems with pH of 8.2 and 6.8, we could find that the ratios (r) of the sizes (d) of the cavities to the diameters (D) of spherical micelles in the systems with pH of 6.8 were larger than those in the solution of pH value at 8.2. This result implied that the formation of the cavity on the surface of the spherical micelle and the variation of the ratios (r) may be controlled by the pH values of the solutions. As expected, the spherical micelles with one cavity were observed as well in the solution with the pH of 4.0 (Fig. 1d and S6b†). Fig. S4d† shows the sizes of these spherical micelles were at the range of 90–200 nm, which was corresponding to the result of the SPM. The average ratio (r = 0.72) of the sizes of the cavities to the diameters of the spherical micelles formed in this condition was larger than the ratio (r = 0.3) observed in the solutions with pH of 6.8, which further demonstrated the influence of the pH values on the morphologies of the nanostructures (shown in Fig. S7†).
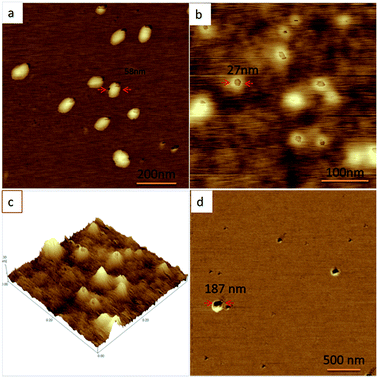 | ||
| Fig. 1 The SPM results of the assembled nanostructures in different pH systems (c = 1 wt%): (a) pH = 8.2; (b) pH = 6.8; (c) 3D structure for (b); (d) pH = 4.0. | ||
In order to understand the mechanism of the formation of the spherical micelles with cavity, the FT-IR experiments were employed to characterize the interactions between the block copolymers. The systems with the pH values at 8.2, 6.8 and 4.0 were dried for 24 h at −40 °C vacuum (ESI 1.5†), respectively. Fig. S8A† shows that the absorption peak of –COOH at the range of 3300–3500 cm−1 increased and the intensities of the absorption peaks decreased with the decrease of the pH values. These results implied the decrease of bound water and the increase of the hydrogen bonding coming from the –COOH with the decrease of the pH values. However, after these freeze-dried samples were dried by infrared rays for 10 min again, the absorption peak of –COOH coming from different pH values decreased and became the same, which implied that the bound water was completely excluded and the hydrogen bonds of –COOH decreased. In Fig. S8B,† the absorption peaks at 1708.6 cm−1 and 1552.4 cm−1 were assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH of carbamates, respectively. These results indicated the extense of the hydrogen bonding of the NH and C
O and NH of carbamates, respectively. These results indicated the extense of the hydrogen bonding of the NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the carbamates. Thus, the formation of the cavity on the surface of the spherical micelle and the variation of the ratios (r) may be considerably due to the collapse of the core of the spherical micelle, probably contributed by the synergistic effects of hydrophobic segments, hydrogen-bonding motifs and the decrease of the water absorbing capacity. The water absorbing capacity, partly coming from the hydrophilic charged segments of the cores, reduced with the decrease of the degree of ionization of the acid groups because of the decrease of the pH values. The extent of the collapse of the cores increased with the decrease of the water absorbing capacity. Thus, the ratios of the sizes of the cavities to the diameters of the spherical micelles gradually increased when the pH value of the systems decreased. Meanwhile, the hydrophobic interactions of the cyclohexyl groups and the hydrogen bonding coming from the carbamates and carboxyl groups, prevented the rearrangement of the blocks of the polymers in the cores and freezed the cores with collapsed cavity, resulting in the formation of the spherical micelles with one cavity on their surfaces. The loss of the solvent in the cores and the hydrogen bonding were all demonstrated to be helpful to the formation of the nanostructures with cavities in other literature.26–29
O of the carbamates. Thus, the formation of the cavity on the surface of the spherical micelle and the variation of the ratios (r) may be considerably due to the collapse of the core of the spherical micelle, probably contributed by the synergistic effects of hydrophobic segments, hydrogen-bonding motifs and the decrease of the water absorbing capacity. The water absorbing capacity, partly coming from the hydrophilic charged segments of the cores, reduced with the decrease of the degree of ionization of the acid groups because of the decrease of the pH values. The extent of the collapse of the cores increased with the decrease of the water absorbing capacity. Thus, the ratios of the sizes of the cavities to the diameters of the spherical micelles gradually increased when the pH value of the systems decreased. Meanwhile, the hydrophobic interactions of the cyclohexyl groups and the hydrogen bonding coming from the carbamates and carboxyl groups, prevented the rearrangement of the blocks of the polymers in the cores and freezed the cores with collapsed cavity, resulting in the formation of the spherical micelles with one cavity on their surfaces. The loss of the solvent in the cores and the hydrogen bonding were all demonstrated to be helpful to the formation of the nanostructures with cavities in other literature.26–29
Notably, the ring-shaped nanostructures (necklaces) were also observed in the system of pH at 6.8 (Fig. 2a, b, S6a and S9a†). The observations of spherical micelles and necklaces in the system of pH at 6.8 were further confirmed by the bimodal distribution of the sizes of the nanostructures shown in Fig. S4c.† The average size of the spherical micelles was about 30 nm, which was corresponding to the lower size part of the DLS result (Fig. S4c†). And the sizes of necklaces were at the range of 200–300 nm, which was corresponding to the higher size part of the DLS result (Fig. S4c†). Excitingly, the ring-shaped nanostructures were composed of a series of globular segments (30 nm). The sizes and shapes of these globular segments were the same as those of the individual spherical micelles with cavities. This phenomenon improved us that the necklaces were formed by the connection of the spherical micelles, which could be also demonstrated by the existence of the imperfect necklaces.
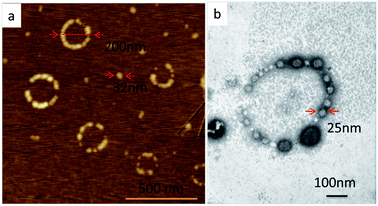 | ||
| Fig. 2 The SPM (a) and TEM (b) results of the assembled nanostructures obtained in the system of pH at 6.8 (c = 1 wt%). | ||
To understand the mechanism of the formation of the necklaces, the effects of the polymer concentrations on the morphologies of the assembled nanostructures were also investigated in detail. For the systems of pH at 6.8, only individual spherical micelles with cavities were observed in the solutions of 0.1 wt% (Fig. 3a) and 0.7 wt% (Fig. 3b). The sizes and shapes of these spherical micelles were the same as those of the individual micelles formed in the solutions of 1 wt%. As the polymer concentration increased to 2 wt%, cylindrical micelles with the cross-sectional diameter of 30 nm were detected (Fig. 3c and S9b†). Fig. S10† shows that the sizes of the cylindrical micelles were at the range of 500–2000 nm. The appearance of some of the cylindrical micelles composed of globular segments (shown in Fig. 3d) implied that the cylindrical micelles were considerably formed by the connection of the spherical micelles as well. However, the sizes and shapes of the nanoparticles assembled in the systems with the pH values at 8.2 and 4.0 remained unchanged when the polymer concentrations varied from 0.1 wt% to 2 wt%. Comparing with the difference of the spherical micelles obtained in different pH systems, the ratios of the sizes of the cavities and the diameters of the spherical micelles gradually increased with the decrease of the pH values, which improved us that lock-and-key interaction was considerably to be one of the major factors for the formation of the hierarchical nanostructures in the system of pH at 6.8. The lock-and-key interaction occurred by the mean of the key particles fitting into the cavity of the lock particles when the sizes of the key particles matched with those of the cavity of the lock particles at certain conditions.30–34 For example, S. Sacanna and coworkers reported the well-defined nanostructures from small clusters to globule-segment chains by the lock-and-key interaction.35
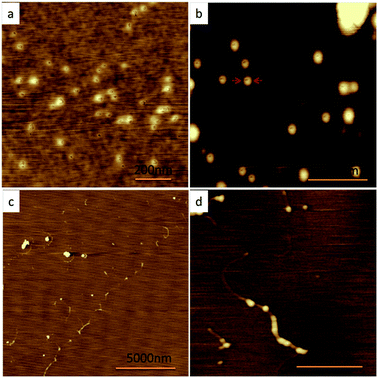 | ||
| Fig. 3 The SPM results of the assembled nanostructures in the solutions of pH at 6.8 with different polymer concentrations: (a) c = 0.1 wt%; (b) c = 0.7 wt%; (c) and (d) c = 2 wt%. | ||
Thus, the formation of the necklaces and the cylindrical micelles could be rationalized as follows. The increase of the Gibbs free energy due to the growth of the polymer concentration drove the spherical micelles to connect to each other, while the lock-and-key interaction guided the directed connection of the spherical micelles. In the system of pH at 6.8, the spherical micelles were both the key particles and the lock particles. The connection of the spherical micelles was accomplished by the insertion of one spherical surface into the cavity of another spherical micelle, which could be further confirmed by the existence of the cavities on the surfaces of the individual spherical micelles and the end globular segments of the curved open-ended segmented chains, and the disappearance of the globular segments of the perfect necklaces. For the systems of pH at 8.2 and 4.0, the sizes of the cavities could not match with the diameters of the spherical micelles, leading to no hierarchical nanostructures.
A novel diblock copolymer with one block alternatively connected with hydrophobic motifs, hydrogen-bonding carbamates and pH-triggered carboxy groups was developed, which showed an exciting pH- and concentration-dependent self-assembly of spherical micelles with cavity, necklaces and cylindrical micelles. These nanostructures could remain stable for more than one month. Spherical micelles with cavity could be prepared by the self-assembly of the diblock copolymer in the aqueous solutions with the pH at 8.2, 6.8 and 4.2, respectively. The ratios of the sizes of the cavities to the diameters of the spherical micelles could be tailored by the pH values. In the systems of pH at 6.8, the diblock copolymer could successively self-assemble into spherical micelles with cavity, necklaces and cylindrical micelles with the increase of the polymer concentration. The formation of the necklaces and cylindrical micelles was considerably due to the coalescence of the spherical micelles with cavity based on the lock-and-key interactions. The strategy to fabricate morphology-controlled nanostructures by designing the molecular structures would probably supply a method to construct the nanomaterials with special properties.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21474065), the National High-tech Research and Development Projects (863) (2013AA06A306), Sichuan Province RST Support Projects ([2015]100-5). We appreciate Xinfeng Cheng, Hanping Li, Xiaopeng Sun, Shuangquan Lai for their aids to our paper.References
- A. Makino, Polym. J., 2014, 46, 783–791 CrossRef CAS.
- S. B. Darling, Prog. Polym. Sci., 2007, 32, 1152–1204 CrossRef CAS.
- S. Jung, W. Kwon, D. Wi, J. Kim, B. J. Ree, Y. Y. Kim, W. J. Kim and M. Ree, Macromolecules, 2016, 49, 1369–1382 CrossRef CAS.
- R. J. Wojtecki and A. Nelson, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 457–472 CrossRef CAS.
- C. G. Palivan, R. Goers, A. Najer, X. Y. Zhang, A. Car and W. Meier, Chem. Soc. Rev., 2016, 45, 377–411 RSC.
- K. Baek, I. Hwang, I. Roy, D. Shetty and K. Kim, Acc. Chem. Res., 2015, 48, 2221–2229 CrossRef CAS PubMed.
- J. M. Hu and S. Y. Liu, Macromol. Chem. Phys., 2015, 216, 591–604 CrossRef CAS.
- J. F. Reuther, D. A. Siriwardane, R. Campos and B. M. Novak, Macromolecules, 2015, 48, 6890–6899 CrossRef CAS.
- Z. M. Hudson, J. S. Qian, C. E. Boott, M. A. Winnik and I. Manners, ACS Macro Lett., 2015, 4, 187–191 CrossRef CAS.
- M. Huo, Q. Q. Ye, H. L. Che, M. Z. Sun, J. Y. Yuan and Y. Wei, Polym. Chem., 2015, 6, 7427–7435 RSC.
- S. Li, J. L. He, M. Z. Zhang, H. R. Wang and P. H. Ni, Polym. Chem., 2016, 7, 1773–1781 RSC.
- R. Qi, Y. Jin, X. F. Cheng, B. Z. Fan, T. B. Sun, S. J. Peng and H. P. Li, Macromol. Rapid Commun., 2015, 36, 1402–1408 CrossRef CAS PubMed.
- Y. Kim, W. Li, S. Shin and M. Lee, Acc. Chem. Res., 2013, 46, 2888–2897 CrossRef CAS PubMed.
- L. S. Shimizu, S. R. Salpage and A. A. Korous, Acc. Chem. Res., 2014, 47, 2116–2127 CrossRef CAS PubMed.
- P. K. Baruah and S. Khan, RSC Adv., 2013, 3, 21202–21217 RSC.
- R. Chapman, M. Danial, M. L. Koh, K. A. Jolliffe and S. Perrier, Chem. Soc. Rev., 2012, 41, 6023–6041 RSC.
- J. X. Yang, B. Fan, J. H. Li, J. T. Xu, B. Y. Du and Z. Q. Fan, Macromolecules, 2016, 49, 367–372 CrossRef CAS.
- E. Obert, M. Bellot, L. Bouteiller, F. Andrioletti, C. Lehen-Ferrenbach and F. Boue, J. Am. Chem. Soc., 2007, 129, 15601–15605 CrossRef CAS PubMed.
- S. H. Kim, F. Nederberg, R. Jakobs, J. P. K. Tan, K. Fukushima, A. Nelson, E. W. Meijer, Y. Y. Yang and J. L. Hedrick, Angew. Chem., Int. Ed., 2009, 48, 4508–4512 CrossRef CAS PubMed.
- N. Chebotareva, P. H. H. Bomans, P. M. Frederik, N. A. J. M. Sommerdijka and R. P. Sijbesma, Chem. Commun., 2005, 4967–4969 RSC.
- S. Dai, P. Ravi and K. C. Tam, Soft Matter, 2008, 4, 435–449 RSC.
- J. R. Lovett, N. J. Warren, L. P. D. Ratcliffe, M. K. Kocik and S. P. Armes, Angew. Chem., Int. Ed., 2015, 54, 1279–1283 CrossRef CAS PubMed.
- N. J. W. Penfold, J. R. Lovett, N. J. Warren, P. Verstraete, J. Smets and S. P. Armes, Polym. Chem., 2016, 7, 79–88 RSC.
- P. Koley and A. Pramanik, J. Mater. Sci., 2014, 49, 2000–2012 CrossRef CAS.
- H. Frisch and P. Besenius, Macromol. Rapid Commun., 2015, 36, 346–363 CrossRef CAS PubMed.
- I. C. Riegel, A. Eisenberg, C. L. Petzhold and D. Samios, Langmuir, 2002, 18, 3358–3363 CrossRef CAS.
- X. Y. Liu, J. S. Kim, J. Wu and A. Eisenberg, Macromolecules, 2005, 38, 6749–6751 CrossRef CAS.
- C. G. Zhang, S. H. Yang, Y. Zhu, R. L. Zhang and X. Y. Liu, Carbohydr. Polym., 2015, 133, 637–643 CrossRef CAS PubMed.
- B. B. Xu, G. G. Gu, C. Feng, X. Jiang, J. H. Hu, G. L. Lu, S. Zhang and X. Huang, Polym. Chem., 2016, 7, 613–624 RSC.
- G. Odriozola, F. Jiménez-Ángeles and M. Lozada-Cassou, J. Chem. Phys., 2008, 129, 111101–111104 CrossRef CAS PubMed.
- F. Jiménez-Ángeles, G. Odriozola and M. Lozada-Cassou, J. Mol. Liq., 2011, 164, 87–100 CrossRef.
- G. E. Eliçabe, J. Colloid Interface Sci., 2011, 357, 82–87 CrossRef PubMed.
- D. J. Beltran-Villegas, L. Colón-Melénde, M. J. Solomon and R. G. Larson, J. Colloid Interface Sci., 2016, 463, 242–257 CrossRef CAS PubMed.
- Y. Wang, Y. F. Wang, X. L. Zheng, G. R. Yi, S. Sacanna, D. J. Pine and M. Weck, J. Am. Chem. Soc., 2014, 136, 6866–6869 CrossRef CAS PubMed.
- S. Sacanna, W. T. M. Irvine, P. M. Chaikin and D. J. Pine, Nature, 2010, 464, 575–578 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, instrument, detailed procedures and characterization of block copolymer and polymer micelles. See DOI: 10.1039/c6ra07799d |
| This journal is © The Royal Society of Chemistry 2016 |
