Theoretical and thermogravimetric study on the thermo-oxidative decomposition of Quinolin-65 as an asphaltene model molecule
Abstract
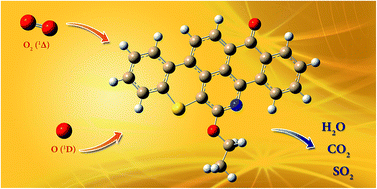
In this study, the thermal oxidation of an asphaltene model molecule, Quinolin-65, was investigated using the density functional theory (DFT) and the second-order Møller–Plesset (MP2) perturbation theory. The reactions studied involved thermal decompositions as well as the interactions between the model molecule and singlet atomic (O1D) and molecular (O21Δ) oxygen. The theoretical study was performed under conditions similar to those of the uncatalyzed thermal oxidation of asphaltenes. A new reaction pathway for the loss of the olefin chain in Quinolin-65 via a 1,3-hydrogen shift mechanism was revealed. Thermogravimetric analysis of Quinolin-65 was also performed and the reaction products were probed by a mass spectrometer. Both the theoretical study and the thermogravimetric analysis concluded that the thermo-oxidative decomposition of Quinolin-65 is a complex multi-step reaction process, which involves different reaction pathways. The thermodynamic parameters obtained in this study showed that the reaction process should start with the loss of the olefin chain in the Quinolin-65 molecule, followed by the oxidation of the aromatic chain, to produce mainly, H2O, CO2, and SO2.

 Please wait while we load your content...
Please wait while we load your content...