Ru-Catalyzed highly site-selective C–H bond arylation of 9-(pyrimidin-2-yl)-9H-carbazole†
K. Harsha Vardhan Reddy*a,
R. Uday Kumara,
V. Prakash Reddya,
G. Satisha,
Jagadeesh Babu Nanubolub and
Y. V. D. Nageswar*a
aMCP Division, Indian Institute of Chemical Technology, Hyderabad 500607, India. E-mail: drharshaindia@gmail.com; dryvdnageswar@gmail.com; Fax: +91-40-27160512
bX-ray Crystallography, Indian Institute of Chemical Technology, Hyderabad 500007, India
First published on 19th May 2016
Abstract
We describe here an efficient ruthenium-catalyzed C–H bond ortho-arylation of 9-(pyrimidin-2-yl)-9H-carbazole by using boronic acids. This methodology exhibits excellent and high site-selectivity, functional group tolerance and was found to give the desired product in moderate to good yields.
Introduction
In recent years, transition-metal-catalyzed C–H bond activation has emerged as an atom economical process with step economy to produce structurally diverse organic molecules. Thus, C–H activation/functionalization emerged as a viable strategy for the synthesis of natural products and other compounds of pharmaceutical and research interest.1–3Carbazoles are heteroaromatic compounds which have attracted considerable attention because carbazole alkaloids are a growing class of natural products that exhibit a variety of biological activities.4 Carbazoles are widely used as building blocks for potential organic semiconductors,5 organic light-emitting diodes,6 and electroluminescent materials.7 Hyellazole, carazomycin and carazostatin are a few carbazole alkaloids that show both antioxidant activity and antibiotic activity (Fig. 1).
In view of the significance of these carbazoles, several methods have been developed. But, the development of efficient methods to modify simple carbazoles is still a major issue, in which selective and direct ortho arylation is especially exciting. To the best of our knowledge, very few methods8 are reported for the ortho-phenylation of carbazoles.
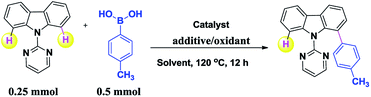
Recently, the use of Ru(II) complexes as inexpensive, readily available, and environmentally friendly catalysts for C–H activation reactions has attracted considerable attention.9 In continuation of our efforts to design and develop novel methodologies in metal-catalyzed C–H activation10 and cross-coupling reactions,11 Herein, we report an efficient Ru-catalyzed regio-selective C–H bond ortho arylation of 9-(pyrimidin-2-yl)-9H-carbazole with boronic acid using Ag2O as oxidant, AgSbF6 as additive in THF (2 ml) at 120 °C for 12 h (Scheme 1).
Results and discussion
Our investigation began by examining several parameters such as the catalyst, additive, oxidants and solvent on Ru-catalyzed regio-selective C–H bond ortho-arylation of 9-(pyrimidin-2-yl)-9H-carbazole (0.25 mmol) with p-tolylboronic acid (0.5 mmol). The results are summarized in Table 1. At first we tried reactions without adding any additive/oxidant, in these cases we did not obtain the desired product (Table 1, entries 1–3). Notably, no product was observed when using various combination of additives and oxidants such as AgSbF6/AgoTf, KPF6/AgBr and KPF6/Ag2O (Table 1, entries 10–12) and AgSbF6/Ag2CO3 provided the desired product in low yield (Table 1, entry 9). Better yield was observed when we used the combination of AgSbF6/Ag2O (Table 1, entry 4). The control experiment showed no detection of the product in the absence Ru catalyst (Table 1, entry 5). We then investigated the effect of a solvent on the reaction. When using DMF as solvent provided unsatisfactory results (Table 1, entry 6). DCE and dioxane solvents provided trace amount of products (Table 1, entries 7–8). Reaction proceeded most satisfactorily in THF as the solvent (Table 1, entry 4). After several trials, we concluded that the best results were obtained with [RuCl2(p-cymene)]2 (5.0 mol%)/Ag2O (1.0 mmol)/AgSbF6 (20 mol%) in THF (2 ml) at 120 °C for 12 h.| Entry | Catalyst | Additive/oxidant | Solvent | Yield (%) |
|---|---|---|---|---|
| a Conditions: 9-(pyrimidin-2-yl)-9H-carbazole (0.25 mmol), p-tolylboronic acid (0.5 mmol) Ru = [RuCl2(p-cymene)]2 (5.0 mol%), additive (20 mol%), oxidant (1.0 mmol), THF (2 ml), 120 °C, 12 h. | ||||
| 1 | Ru | — | THF | 0 |
| 2 | Ru | AgSbF6/— | THF | 0 |
| 3 | Ru | —/Ag2O | THF | 0 |
| 4 | Ru | AgSbF6/Ag2O | THF | 71 |
| 5 | — | AgSbF6/Ag2O | THF | 0 |
| 6 | Ru | AgSbF6/Ag2O | DMF | 45 |
| 7 | Ru | AgSbF6/Ag2O | DCE | Trace |
| 8 | Ru | AgSbF6/Ag2O | Dioxane | 10 |
| 9 | Ru | AgSbF6/Ag2CO3 | THF | 48 |
| 10 | Ru | AgSbF6/Ag2OTf | THF | 0 |
| 11 | Ru | KPF6/AgBr | THF | 0 |
| 12 | Ru | KPF6/Ag2O | THF | 0 |
We next explored the scope of the ruthenium-catalyzed C–H bond arylation of 9-(pyrimidin-2-yl)-9H-carbazole 1 with aryl boronic acid 2 using the optimized conditions (Table 2). A variety of aryl boronic acid with different functional groups were tested. Various kinds of functional groups, such as –Me, –OMe, CF3, –F, –Cl, –I and even –CHO were well tolerated.
| a Conditions: 9-(pyrimidin-2-yl)-9H-carbazole (0.25 mmol), p-tolylboronic acid (0.5 mmol) Ru = [RuCl2(p-cymene)]2 (5.0 mol%), additive (20 mol%), oxidant (1.0 mmol), THF (2 ml), 120 °C, 12 h. |
|---|
 |
Varying both electron-rich as well as electron-deficient on 2 did not significantly influence the reaction; as a result, the desired products 3b and 3c were obtained in 71 and 61% yields, respectively.
All products were characterized by 1H and 13C NMR and mass spectra. However, a small amount di ortho-arylation side product detected in few cases. The structure of the product (3b) was confirmed by X-ray crystallographic analysis (Fig. 2).12
Conclusions
In conclusion, we have developed a general, mild and efficient procedure of Ru-catalyzed highly regioselective ortho-arylation of 9-(pyrimidin-2-yl)-9H-carbazoles with substituted aromatic and heteroaromatic boronic acids in the presence of AgSbF6 and Ag2O. This protocol exhibits a broad substrate scope and this reaction tolerates a wide range of functional groups. Moreover, the products could also be further transformed to diverse important derivatives.Experimental section
General experimental procedure for the synthesis of 1-phenyl-9-(pyrimidin-2-yl)-9H-carbazole
To a mixture of 9-(pyrimidin-2-yl)-9H-carbazole (0.25 mmol), phenylboronic acid (0.5 mmol), [RuCl2(p-cymene)]2 (5.0 mol%), Ag2O (1.0 mmol) and AgSbF6 (20 mol%), THF (2 ml) was added and thereafter, the reaction mixture was stirred at 120 °C for 12 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was extracted with ethyl acetate. The combined organic phase was washed with brine and dried with anhydrous Na2SO4. The solvent was evaporated under vacuum to give the crude product which was purified by column chromatography on silica gel. The purity of the product was confirmed by 1H NMR, 13C NMR and mass spectra. 3a (69% yield) 1H NMR (300 MHz, CDCl3): δ 8.31 (d, J = 4.8 Hz, 2H), 8.12 (dd, J = 8.5, 3.4 Hz, 3H), 7.51–7.22 (m, 6H), 7.14–7.02 (m, 3H), 6.82 (t, J = 4.8 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 157.1, 140.7, 128.5, 127.9, 127.4, 126.6, 125.9, 122.2, 121.8, 119.9, 119.1, 116.8, 112.0; ESI-MS: m/z 322 [M + 1].Acknowledgements
The authors thank the Council of Scientific and Industrial Research (CSIR), New Delhi for financial assistance.References
- M. Beller and C. Bolm, Transition Metals for Organic Synthesis, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed.
- J. Tsuji, Palladium Reagents and Catalysts, Wiley, Chichester, 2nd edn, 2004 Search PubMed.
- L. Ackermann, Modern Arylation Methods, Wiley-VCH, Weinheim, 2009 Search PubMed.
- (a) H. J. Knolker and K. R. Reddy, Chem. Rev., 2002, 102, 4303 CrossRef PubMed; (b) P. T. Gallagher, Science of Synthesis, Thieme, Stuttgart, 2000, vol. 10, p. 693 Search PubMed; (c) H. J. Knolker, in Advances in Nitrogen Heterocycles, ed. C. J. Moody, JAI, Greenwich, 1995, vol. 1, p. 173 Search PubMed; (d) E. Brunner and C. Jutz, Methoden Org. Chem. (Houben–Weyl), 4th edn, 1952, vol. E6a, p. 922 Search PubMed; (e) C. J. Moody, Synlett, 1994, 681 CrossRef CAS; (f) D. P. Chakraborty, in The Alkaloids, ed. A. Bossi, Academic Press, New York, 1993, vol. 44, p. 257 Search PubMed; (g) D. P. Chakraborty and S. Roy, Prog. Chem. Org. Nat. Prod., 1991, 57, 71 CAS.
- S. Walkim, J. Bouchard, N. Blouin, A. Michaud and M. Leclerc, Org. Lett., 2004, 6, 3413 CrossRef PubMed.
- A. V. Dijken, J. J. A. M. Bastiaansen, N. M. M. Kiggen, B. M. W. Langeveld, C. Rothe, A. Monkman, I. Bach, P. Stossel and K. Brunner, J. Am. Chem. Soc., 2004, 126, 7718 CrossRef PubMed.
- K. R. J. Thomas, J. T. Lin, Y. T. Tao and C. W. Ko, J. Am. Chem. Soc., 2001, 123, 9404 CrossRef CAS PubMed.
- (a) T. Ohta, S. Miyake and K. Shudo, Tetrahedron Lett., 1985, 26, 5811 CrossRef CAS; (b) K. Haga, K. Iwaya and R. Kaneko, Bull. Chem. Soc. Jpn., 1986, 59, 803 CrossRef CAS; (c) J. H. Chu, C. C. Wu, D. H. Chang, Y. M. Lee and M. J. Wu, Organometallics, 2013, 32, 272 CrossRef CAS.
- (a) L. Ackermann, A. V. Lygin and N. Hofmann, Angew. Chem., Int. Ed., 2011, 50, 6379 CrossRef CAS PubMed; (b) L. Ackermann, A. V. Lygin and N. Hofmann, Org. Lett., 2011, 13, 3278 CrossRef CAS PubMed; (c) L. Ackermann and S. Fenner, Org. Lett., 2011, 13, 6548 CrossRef CAS PubMed; (d) T. Ueyama, S. Mochida, T. Fukutani, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2011, 13, 706 CrossRef CAS PubMed; (e) Y. Hashimoto, T. Ueyama, S. Mochida, T. Fukutani, K. Hirano, T. Satoh and M. Miura, Chem. Lett., 2011, 40, 1165 CrossRef CAS; (f) P. Kishor and M. Jeganmohan, Org. Lett., 2011, 13, 6144 CrossRef PubMed; (g) C. G. Ravi Kiran and M. Jeganmohan, Eur. J. Org. Chem., 2012, 417 Search PubMed; (h) C. G. Ravi Kiran and M. Jeganmohan, Chem. Commun., 2012, 48, 2030 RSC; (i) L. Ackermann, L. Wang and A. V. Lygin, Chem. Sci., 2012, 3, 177 RSC; (j) L. Ackermann and A. V. Lygin, Org. Lett., 2011, 13, 3332 CrossRef CAS PubMed; (k) J. Hubrich, T. Himmler, L. Rodefeld and L. Ackermann, Adv. Synth. Catal., 2015, 357, 474 CrossRef CAS.
- (a) K. H. V. Reddy, G. Satish, V. P. Reddy, B. S. P. A. Kumar and Y. V. D. Nageswar, RSC Adv., 2012, 2, 11084 RSC; (b) G. Satish, K. H. V. Reddy, B. S. P. Anil, J. Shankar, R. U. Kumar and Y. V. D. Nageswar, Tetrahedron Lett., 2014, 40, 5533 CrossRef.
- (a) G. Satish, K. H. V. Reddy, K. Ramesh and K. Karnakar, Tetrahedron Lett., 2012, 53, 2518 CrossRef CAS; (b) K. Swapna, S. N. Murthy, M. T. Jyothi and Y. V. D. Nageswar, Org. Biomol. Chem., 2011, 9, 5978 RSC; (c) K. Swapna, S. N. Murthy, M. T. Jyothi and Y. V. D. Nageswar, Org. Biomol. Chem., 2011, 9, 5989 RSC; (d) K. H. V. Reddy, V. P. Reddy, J. Shankar, B. Madhav and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 2679 CrossRef CAS; (e) K. H. V. Reddy, V. P. Reddy, J. Shankar and Y. V. D. Nageswar, Synlett, 2011, 9, 1268 Search PubMed; (f) K. H. V. Reddy, A. A. Kumar, G. Kranthi and Y. V. D. Nageswar, Beilstein J. Org. Chem., 2011, 7, 886 CrossRef CAS PubMed; (g) K. H. V. Reddy, G. Satish, K. Ramesh, K. Karnakar and Y. V. D. Nageswar, Chem. Lett., 2012, 41, 585 CrossRef CAS; (h) B. S. P. A. Kumar, K. H. V. Reddy, B. Madhav, K. Ramesh and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 4595 CrossRef; (i) K. H. V. Reddy, G. Satish, K. Ramesh, K. Karnakar and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 3061 CrossRef CAS; (j) G. Satish, K. H. V. Reddy, K. Ramesh and B. S. P. A. Kumar, Tetrahedron Lett., 2014, 55, 2596 CrossRef CAS; (k) B. S. P. Anil Kumar, K. H. V. Reddy, K. Karnakar, G. Satish and Y. V. D. Nageswar, Tetrahedron Lett., 2015, 56, 1968 CrossRef CAS; (l) K. H. V. Reddy, B. S. P. Anil Kumar, V. P. Reddy, R. U. Kumar and Y. V. D. Nageswar, RSC Adv., 2014, 4, 45579 RSC; (m) B. S. P. A. Kumar, K. H. V. Reddy, G. Satish, R. U. Kumar and Y. V. D. Nageswar, RSC Adv., 2014, 4, 60652 RSC.
- X-ray data for the compound (3b) were collected at room temperature using a Bruker Smart Apex CCD diffractometer with graphite monochromated MoKα radiation (λ = 0.71073 Å) with ω-scan method. Preliminary lattice parameters and orientation matrices were obtained from four sets of frames. Unit cell dimensions were determined using 9274 reflections for 3b data. Integration and scaling of intensity data were accomplished using SAINT program. The structures were solved by Direct Methods using SHELXS97 and refinement was carried out by full-matrix least-squares technique using SHELXL97. Anisotropic displacement parameters were included for all non-hydrogen atoms. All H atoms were positioned geometrically and treated as riding on their parent C atoms, with C–H distances of 0.93–0.97 Å, and with Uiso (H) = 1.2Ueq. (C) or 1.5Ueq. for methyl atoms. Crystal data for the compound 3b: C23H17N3, M = 335.40, colourless block, 0.49 × 0.33 × 0.28 mm3, monoclinic, space group P21/n (no. 14), a = 9.5942(6), b = 11.3101(7), c = 16.4330(10) Å, β = 96.6930(10)°, V = 1771.01(19) Å3, Z = 4, Dc = 1.258 g cm−3, F000 = 704, CCD area detector, MoKα radiation, λ = 0.71073 Å, T = 293(2) K, 2θmax = 50.0°, 16640 reflections collected, 3128 unique (Rint = 0.0178), Final GooF = 1.035, R1 = 0.0377, wR2 = 0.1041, R indices based on 2784 reflections with I > 2σ(I) (refinement on F2), 236 parameters, μ = 0.075 mm−1. CCDC 1059104 contains the supplementary crystallographic data for this paper.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1059104. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra09825h |
| This journal is © The Royal Society of Chemistry 2016 |