Spectroscopic and microscopic investigation of the effects of bacteria on dental implant surfaces
Abstract
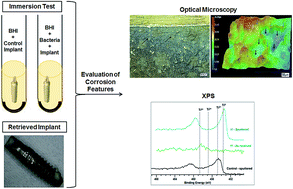
The surface morphology and chemical composition of commercially pure titanium dental implants and healing abutments exposed in vitro or in vivo to oral bacteria were studied. Implants exposed in vitro to media containing S. mutans and healing abutment retrievals were used to evaluate the effect of early bacterial colonizers. Another dental implant retrieval that failed due to peri-implantitis and implants exposed to an in vitro culture of P. gingivalis were analyzed to assess the effect of late stage colonizing bacteria. Using optical microscopy and X-ray photoelectron spectroscopy, it was determined that both S. mutans and P. gingivalis adversely affected the implant surface, with the early colonizing S. mutans creating conditions leading to more severe corrosion features being observed, including pitting and surface discoloration. Overall, this study demonstrated the ability of bacteria in the oral environment to solely induce changes in the oxidation states of titanium and subsequent corrosion of the implant surface, even in the absence of mechanical loads.

 Please wait while we load your content...
Please wait while we load your content...