Recent trends in nano-based drug delivery systems for efficient delivery of phytochemicals in chemotherapy
Abstract
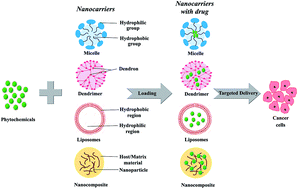
The advent of nanotechnology has revolutionized various scientific inventions, out of which the debut of nanomedicine is outstanding. Especially, research has embarked on nano-drug delivery for treating cancer. Natural compounds present in plants, namely phytochemicals, have been extensively exploited for their anticancer properties. Despite their excellent anticancer abilities, phytochemicals are limited by their low water solubility and poor bioavailability. However, the field of nanotechnology has overcome these limitations. This review focusses on various methods of nano-drug delivery of phytochemicals against the killer disease, cancer. Common carriers that were employed ranged from micelles, with a polymeric base, to dendrimers, liposomes and nanoparticles. The phytochemicals were found to become more soluble when delivered by the nanocarriers and exhibited a remarkable effect on the cancer cells, compared to their free form. More interestingly, the half-maximal dose of the phytochemical was reduced significantly when it was delivered by the nanocarrier. On the whole, this review encourages the idea of “cancer-nanotechnology” after in-depth clinical studies on these phytochemical-loaded nanocarriers. Moreover, it will epitomize the nanocarriers as a crusader in improving cancer chemotherapy by reducing undesired effects and will invigorate site-specific drug delivery.

 Please wait while we load your content...
Please wait while we load your content...