Three-component 2-aryl substituted benzothiophene formation under transition-metal free conditions†
Pengcheng Jiang,
Xingzong Che,
Yunfeng Liao,
Huawen Huang* and
Guo-Jun Deng*
Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan 411105, China. E-mail: hwhuang@xtu.edu.cn; gjdeng@xtu.edu.cn; Fax: +86-0731-5829-2251; Tel: +86-0731-5829-8280
First published on 20th April 2016
Abstract
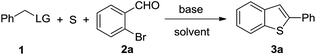
A base-mediated 2-aryl substituted benzothiophene formation from 2-bromobenzene aldehydes, benzylic esters and elemental sulfur under transition-metal-free conditions is described. Various 2-aryl substituted benzothiophene were efficiently obtained under mild conditions.
Benzothiophene derivatives are basic skeletons frequently found in electronic materials as well as various biologically active molecules.1 The benzothiophene motifs also can be found in a number of medicinally important molecules such as raloxifene (selective estrogen receptor modulators), arzoxifene (antitubulin agents) and zileuton (selective inhibitor of 5-lipoxygenase) (Fig. 1).2 Consequently, the development of convenient synthetic methods to these sulfur-containing heterocycles has received much attention during the past decades. However, compared with other heteroaromatic rings, especially its analogues benzofuran, indole and benzothiazole, efficient methods for the preparation of benzothiophene skeleton are rather limited. The most common approach is based on the intramolecular cyclization procedures.3 α-Arylthioketones, o-alkynyl (or ynol or alkenyl) benzenthiols, and alkynyl(aryl)thioethers are frequently used as the substrates for this transformation. Other methods recently developed include metal-promoted annulation approaches with highly functionalized thiophenols and photocatalytic synthesis of benzothiophenes with visible light from o-methylthio-arenediazonium salts and alkynes.4 These reactions are proved efficient and selective, but prefunctionalized thiophenols had to be synthesized before the cyclization reactions. To overcome this limitation, Li et al. developed an oxygen-triggered intermolecular cyclization of thiophenols with electron-deficient alkynes to provide a more direct approach to benzothiophene skeleton.5 Alternatively, the transition-metal catalyzed cross-coupling reactions of metalated benzothiophenes and halogenated arenes could provide an efficient approach for aryl-substituted benzothiophene.6 More recently, the direct arylation of benzothiophene has emerged as an attractive approach for the preparation of substituted benzothiophenes by skipping prefunctionalization of the substrates.7 Transition metals such as palladium and copper were proved to be effective catalysts for these reactions.
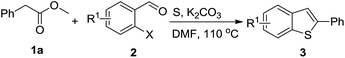
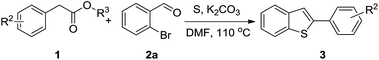
In recent years, sulfur-containing heterocycle formation using cheap, non-volatile and readily available elemental sulfur as the sulfur source has attracted great attention.8 Zhou and co-workers developed a copper-catalyzed multicomponent reaction for the synthesis of 2-substituted benzothiazoles from 2-iodo-anilines, aldehydes, and elemental sulfur (Scheme 1a).8a Using elemental sulfur as the sulfur source for the synthesis of benzothiazoles, Nguyen8b,c and Han8d independently disclosed a concise reaction by redox condensation between o-halonitrobenzenes and methylarenes under catalyst-free conditions (Scheme 1b). Based on our previous efforts of sulfur-containing heterocycle synthesis from elemental sulfur,9 we suspect the coupling of 2-halobenzaldehydes, methylarenes, and elemental sulfur could form aryl sulfide intermediate I, which then proceed through condensation and subsequent elimination to afford benzothiophenes (Scheme 1c). Herein, we report such a three-component approach for the 2-aryl benzothiophene formation from 2-halobenzaldehydes, benzylic esters and sulfur under air.
 | ||
| Scheme 1 Sulfur-containing heterocycle formation using elemental sulfur. X = F, Cl, Br; LG = leaving group. | ||
To obtain the optimized reaction conditions, we began our study by examining a series of methylarenes with a range of leaving groups. Based on previous report, toluene even 4-methyl pyridine were firstly tested, and unfortunately the desired product was not observed (Table 1, entry 1). Moreover, benzylamine was also found unproductive (entry 2). To our delight, when we tested benzyl chloride as the substrate, the desired benzothiophene 3a was formed, albeit in a low yield. Since the reaction is proposed to be base-initiated transformation, the reactants with electron-withdrawing leaving group such as carboxyl and ester were then screened. While phenylacetic acid gave a low yield of 3a, methyl phenylacetate enhanced the yield to 60% (entry 5). The promising result encouraged us to screen the effect of base. Gratefully, among the inorganic bases investigated, K2CO3 showed the best efficiency to give the corresponding product in 92% yield (entry 6). Cs2CO3 is also a proper base to promote this kind of transformation to afford 3a in 88% yield (entry 7). However, the use of strong base such KOH significantly decreased the yield (entry 8). In addition, organic bases such as pyridine and triethyl amine were not effective for this reaction (entries 9–10). Solvent plays an important role in this reaction, and the use of DMA as the solvent decreased the yield to 78% (entry 11). Other organic solvents such as toluene and NMP were not suitable media (entries 12–13). No product was observed when the reaction was carried out in aqueous media (entry 14). The reaction yield was slightly improved to 97% when the temperature was decreased to 130 °C (entry 15). High yield could be remained when the reaction temperature was further decreased to 110 °C (entry 16).
| Entry | LG | Base | Solvent | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.3 mmol), 2a (0.2 mmol), S (0.8 mmol), base (0.6 mmol), solvent (0.5 mL), 150 °C, 16 h, under air unless otherwise noted.b GC yield based on 2a using dodecane as an internal standard.c At 130 °C.d At 110 °C. | ||||
| 1 | H | Na2CO3 | DMF | ND |
| 2 | NH2 | Na2CO3 | DMF | ND |
| 3 | Cl | Na2CO3 | DMF | 12 |
| 4 | COOH | Na2CO3 | DMF | 18 |
| 5 | COOMe | Na2CO3 | DMF | 60 |
| 6 | COOMe | K2CO3 | DMF | 92 |
| 7 | COOMe | Cs2CO3 | DMF | 88 |
| 8 | COOMe | KOH | DMF | 14 |
| 9 | COOMe | Et3N | DMF | ND |
| 10 | COOMe | Pyridine | DMF | ND |
| 11 | COOMe | K2CO3 | DMA | 78 |
| 12 | COOMe | K2CO3 | NMP | 30 |
| 13 | COOMe | K2CO3 | Toluene | 16 |
| 14 | COOMe | K2CO3 | H2O | ND |
| 15c | COOMe | K2CO3 | DMF | 97 |
| 16d | COOMe | K2CO3 | DMF | 96 |
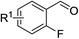
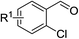
With the optimized reaction conditions in hand, the substrate scope with respect to 2-halobenzaldehydes was explored (Table 2). Good yields were obtained when methoxy and chloro substituents were located at the para position of the bromine atom (Table 2, entries 2–3). To our delight, 2-fluorobenzaldehydes also reacted with 1a and sulfur to give the corresponding products (entries 4–8). When a trifluoromethyl group was located at the para position of the fluoro atom, the corresponding product 3e was obtained in 87% yield (entry 6). However, a much lower yield was obtained when 2,6-difluorobenzaldehyde (2g) was employed (entry 7). Moderate yield could be achieved when 2,3-difluorobenzaldehyde (2h) was used as the substrate (entry 8). We also investigated the reaction of 2-chlorobenzaldehyde (2i) with 1a and sulfur under the same reaction conditions. High yield could be achieved when slightly increasing the reaction temperature to 120 °C (entry 9). The desired product 3i was obtained in 82% yield when 2,6-dichlorobenzaldehyde (2k) was used (entry 10). Similarly, much lower yield was obtained when the chloro substituent was located at the ortho position of the aldehyde group (entry 11).
| Entry | Benzaldehyde | Product | Yieldb (%) | |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2 (0.2 mmol), S (0.8 mmol), K2CO3 (0.6 mmol), DMF (0.5 mL), 110 °C, 16 h under air.b Isolated yield based on 2.c At 120 °C. | ||||
 |
||||
| 1 | R1 = H | 2a | 3a | 89 |
| 2 | R1 = 5-OCH3 | 2b | 3b | 73 |
| 3 | R1 = 5-CI | 2c | 3c | 84 |
 |
||||
| 4 | R1 = H | 2d | 3a | 72 |
| 5 | R1 = 5-Br | 2e | 3d | 74 |
| 6 | R1 = 5-CF3 | 2f | 3e | 87 |
| 7 | R1 = 6-F | 2g | 3f | 30 |
| 8 | R1 = 3-F | 2h | 3g | 61 |
 |
||||
| 9c | R1 = H | 2i | 3a | 84 |
| 10c | R1 = 6-CI | 2j | 3h | 38 |
| 11c | R1 = 3-CI | 2k | 3i | 82 |
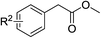
To further examine the scope and limitations of the reaction, various 2-arylacetate esters (1) were treated with 2-bromobenzaldehyde (2a) and sulfur under the optimized conditions (Table 3). Lower yields were obtained when electron-donating groups such as methoxy and amine were presented at the para position (Table 3, entries 1 and 5). Moderate yields were obtained when electron-withdrawing halogen and nitro groups were presented (entries 2–6). The position of substituents on phenyl ring of methyl phenylacetates significantly affected the reaction yield (entries 7–9). When methyl 2-(2,3-dichlorophenyl)acetate (1k) and methyl 2-(2,4-dichlorophenyl)acetate (1l) were used, the corresponding products 3s and 3t were obtained in 82% and 79% yields, respectively (entries 10 and 11). The reaction of steric substrate such as methyl 2-(naphthalen-2-yl)acetate (1m) with 2a and sulfur still afforded the product 3u in 63% yield (entry 12). Notably, pyridyl ester reacted well in this reaction to afford the product 3v in 85% yield (entry 13). In addition, ethyl and tert-butyl phenylacetates could also react with 2a and sulfur to provide the products 3a in good yields (entries 14 and 15).
| Entry | 2-Arylacetate | Product | Yieldb (%) | |
|---|---|---|---|---|
| a Conditions: 1 (0.3 mmol), 2a (0.2 mmol), S (0.8 mmol), K2CO3 (0.6 mmol), DMF (0.5 mL), 110 °C, 16 h under air.b Isolated yield based on 2a. | ||||
 |
||||
| 1 | R2 = 4-OCH3 | 1b | 3j | 28 |
| 2 | R2 = 4-F | 1c | 3k | 51 |
| 3 | R2 = 4-CI | 1d | 3l | 43 |
| 4 | R2 = 4-Br | 1e | 3m | 48 |
| 5 | R2 = 4-NH2 | 1f | 3n | 30 |
| 6 | R2 = 4-NO2 | 1g | 3o | 50 |
| 7 | R2 = 3-OCH3 | 1h | 3p | 77 |
| 8 | R2 = 3-F | 1i | 3q | 61 |
| 9 | R2 = 2-Cl | 1j | 3r | 73 |
| 10 | R2 = 2,3-Cl2 | 1k | 3s | 82 |
| 11 | R2 = 2,4-Cl2 | 1l | 3t | 79 |
| 12 |  |
1m | 3u | 63 |
| 13 | 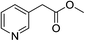 |
1n | 3v | 85 |
| 14 | 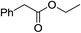 |
1o | 3a | 78 |
| 15 | 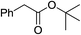 |
1p | 3a | 55 |
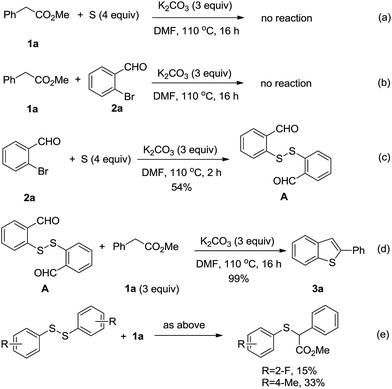
We reason that the formation of 2-substituted benzothiophene compounds could be attributed to a tandem nucleophilic substitution and intramolecular cyclization process (Scheme 1c). To probe such a procedure, a serious control experiments were conducted. While benzylic ester 1a was completely recovered when the reaction was performed in the absence of 2a (Scheme 2a), the combination of 1a and 2a in the absence of sulfur was also unfruitful, with the two reactants recovered (Scheme 2b). Moreover, 2-bromobenzene aldehyde (2a) could react with sulfur to form disulfide A in the absence of 1a (Scheme 2c). Then, the intermediate A could transfer into the target benzothiophene 3a with almost full conversation (Scheme 2d). Finally, to demonstrate the coupling of disulfide and 1a, two disulfides without carbonals were subjected to the system (Scheme 2e). Both the 2-F- and 4-Me-reactants could afford the coupling products, albeit in low yields (GC-MS analysis). Those observations along with several reported works10 give the basis for the mechanistic insight of the present reaction. As shown in Scheme 3, the base-promoted coupling of 2-bromobenzene aldehyde with elemental sulfur, which would be activated by the base/heat conditions,8 would be the first step, which forms disulfide intermediate A. Subsequently, the nucleophilic attack of 1a to A occurs to give intermediate B. Intramolecular aldol-type reaction of B affords intermediate C. Then, the demethoxycarbonylation promoted by base generates the intermediate E, which undergoes dehydration to afford the final 3a.10a,b This procedure releases 2-formylbenzenethiolate D, which could be readily oxidized into disulfide A under air.
Conclusions
In summary, we have developed a novel approach for 2-aryl substituted benzothiophene formation from 2-bromobenzene aldehydes, benzylic esters and elemental sulfur under transition-metal-free conditions. The use of potassium carbonate significantly improved the reaction yield. Functional groups such as halogen, trifluoromethyl and nitro were well tolerated under the optimized reaction conditions. Since elemental sulfur is cheap and stable, this three-component approach affords an efficient route for the rapid synthesis of 2-aryl substituted benzothiophenes in the absence of transition-metal-catalyst. The scope, mechanism, and synthetic application of this reaction are under investigation.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21372187, 21572194), the Program for Innovative Research Cultivation Team in University of Ministry of Education of China (1337304) and the Hunan Provincial Innovative Foundation for Postgraduate (CX2014B264).Notes and references
-
(a) C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2012, 112, 2208 CrossRef CAS PubMed
; (b) L. Li, M.-C. Mathieu, D. Denis, A. G. Therien and Z. Wang, Bioorg. Med. Chem. Lett., 2011, 21, 734 CrossRef CAS PubMed
; (c) S. Vangveravong, M. Taylor, J. Xu, J. Cui, W. Calvin, S. Babic, R. R. Luedtke and R. H. Mach, Bioorg. Med. Chem., 2010, 18, 5291 CrossRef CAS PubMed
; (d) S. Wang, R. Beck, T. Blench, A. Burd, S. Buxton, M. Malic, T. Ayele, S. Shaikh, S. Chahwala, C. Chander, R. Holland, S. Merette, L. Zhao, M. Blackney and A. Watts, J. Med. Chem., 2010, 53, 1465 CrossRef CAS PubMed
; (e) I. Fouad, Z. Mechbal, K. I. Chane-Ching, A. Adenier, F. Maurel, J.-J. Aaron, P. Vodicka, K. Cernovska, V. Kozmik and J. Svoboda, J. Mater. Chem., 2004, 14, 711 RSC
; (f) K. H. Jung, K. H. Kim, D. H. Lee, D. S. Jung, C. E. Park and D. H. Choi, Org. Electron., 2010, 11, 1584 CrossRef CAS
; (g) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed
.
-
(a) Z. Qin, I. Kasrati, E. P. Chandrasena, H. Liu, P. Yao, P. A. Petukhov, J. L. Bolton and G. R. J. Thatcher, J. Med. Chem., 2007, 50, 2682 CrossRef CAS PubMed
; (b) C.-N. Hsiao and T. Kolasa, Tetrahedron Lett., 1992, 33, 2629 CrossRef CAS
.
-
(a) B. Gabriele, R. Mancuso, E. Lupinacci, L. Veltri, G. Salerno and C. Carfagna, J. Org. Chem., 2011, 76, 8277 CrossRef CAS PubMed
; (b) Y.-S. Kim, S. H. Kwak and Y.-D. Gong, ACS Comb. Sci., 2015, 17, 365 CrossRef CAS PubMed
; (c) K. Inamoto, Y. Arai, K. Hiroya and T. Doi, Chem. Commun., 2008, 5529 RSC
; (d) I. Nakamura, T. Sato and Y. Yamamoto, Angew. Chem., Int. Ed., 2006, 45, 4473 CrossRef CAS PubMed
; (e) M. C. Willis, D. Taylor and A. T. Gillmore, Tetrahedron, 2006, 62, 11513 CrossRef CAS
.
-
(a) D. P. Hari, T. Hering and B. König, Org. Lett., 2012, 14, 5334 CrossRef CAS PubMed
; (b) Z. Duan, S. Ranjit and X. Liu, Org. Lett., 2010, 12, 2430 CrossRef CAS PubMed
.
- K. Liu, F. Jia, H. Xi, Y. Li, X. Zheng, Q. Guo, B. Shen and Z. Li, Org. Lett., 2013, 15, 2066 CrossRef PubMed
.
-
(a) B. Join, T. Yamamoto and K. Itami, Angew. Chem., Int. Ed., 2009, 48, 3644 CrossRef CAS PubMed
; (b) H.-Q. Do, R. M. K. Khan and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 15185 CrossRef CAS PubMed
; (c) C. S. Bryan, J. A. Braunger and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 7064 CrossRef CAS PubMed
; (d) K. Takimiya, H. Ebata, K. Sakamoto, T. Izawa, T. Otsubo and Y. Kunugi, J. Am. Chem. Soc., 2006, 128, 12604 CrossRef CAS PubMed
; (e) F. Zeng and H. Alper, Org. Lett., 2013, 15, 2686 Search PubMed
.
-
(a) X. Qin, D. Sun, Q. You, Y. Cheng, J. Lan and J. You, Org. Lett., 2015, 17, 1762 CrossRef CAS PubMed
; (b) C.-Y. He, S. Fan and X. Zhang, J. Am. Chem. Soc., 2010, 132, 12850 CrossRef CAS PubMed
; (c) V. P. Reddy, R. Qiu, T. Iwasaki and N. Kambe, Org. Lett., 2013, 15, 1290 CrossRef CAS PubMed
; (d) J. Dong, Z. Long, F. Song, N. Wu, Q. Guo, J. Lan and J. You, Angew. Chem., Int. Ed., 2013, 52, 580 CrossRef CAS PubMed
; (e) N. Kuhl, M. N. Hopkinson and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 8230 CrossRef CAS PubMed
; (f) J. Wencel-Delord, C. Nimphius, H. Wang and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 13001 CrossRef CAS PubMed
; (g) H.-Q. Do and O. Daugulis, J. Am. Chem. Soc., 2011, 133, 13577 CrossRef CAS PubMed
; (h) Y. Huang, D. Wu, J. Huang, Q. Guo, J. Li and J. You, Angew. Chem., Int. Ed., 2014, 53, 12158 CrossRef CAS PubMed
; (i) X. Qin, X. Li, Q. Huang, H. Liu, D. Wu, Q. Guo, J. Lan, R. Wang and J. You, Angew. Chem., Int. Ed., 2015, 54, 7167 CrossRef CAS PubMed
; (j) P. Xi, F. Yang, S. Qin, D. Zhao, J. Lan, G. Gao, C. Hu and J. You, J. Am. Chem. Soc., 2010, 132, 1822 CrossRef CAS PubMed
.
-
(a) H. Deng, Z. Li, F. Ke and X. Zhou, Chem.–Eur. J., 2012, 18, 4840 CrossRef CAS PubMed
; (b) T. B. Nguyen, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2013, 15, 4218 CrossRef CAS PubMed
; (c) T. B. Nguyen, L. Ermolenko, P. Retailleau and A. Al-Mourabit, Angew. Chem., Int. Ed., 2014, 53, 13808 CrossRef CAS PubMed
; (d) Y. Tong, Q. Pan, Z. Jiang, D. Miao, X. Shi and S. Han, Tetrahedron Lett., 2014, 55, 5499 CrossRef CAS
.
- Y. Liao, Y. Peng, H. Qi, G.-J. Deng, H. Gong and C.-J. Li, Chem. Commun., 2015, 51, 1031 RSC
.
-
(a) S. R. Chemburkar, D. G. Anderson and R. E. Reddy, Synth. Commun., 2010, 40, 1887 CrossRef CAS
; (b) S. W. Landvatter, Heterocycles, 1996, 43, 1189 CrossRef CAS
; (c) C. F. Roberts and R. C. Hartley, J. Org. Chem., 2004, 69, 6145 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07730g |
| This journal is © The Royal Society of Chemistry 2016 |