Thinning of n-layer MoS2 by annealing a palladium film under vacuum†
Ya Dengab,
Minjiang Chenab,
Jian Zhangab,
Xiao Huab,
Yun Zhaoa,
Jean Pierre Nshimiyimanaab,
Xiannian Chiab,
Gu Houab,
Weiguo Chu*a and
Lianfeng Sun*a
aCAS Key Laboratory of Nanosystem and Hierarchical Fabrication, CAS Center for Excellence in Nanoscience, National Centre for Nanoscience and Technology, Beijing 100190, China. E-mail: wgchu@nanoctr.cn; slf@nanoctr.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 18th May 2016
Abstract
When Pd is thermally evaporated onto n-layer MoS2, a uniform film is formed on the surface. If Pd/n-layer MoS2 is annealed at high temperature under vacuum, thinning of n-layer MoS2 is observed. By controlling the thickness of the Pd film and annealing temperature, one or two top layers of n-layer MoS2 can be removed during the annealing process. The thinning method presented in this work benefits the future design and fabrication of MoS2-based devices.
Compared to graphene,1–4 which is a zero-gap material and limits on/off ratios of graphene-based field effect transistors, semiconducting two-dimensional materials have attracted great interest in electrical and photoelectric devices. As a typical transition-metal dichalcogenide semiconductor, molybdenum disulfide (MoS2) exhibits unique physical, optical and electrical properties,5–10 which have attracted more attention recently. One remarkable property of MoS2 is the indirect-to-direct band gap transition for bulk to monolayer MoS2.5 Therefore, it is important to prepare and control the thickness of n-layer MoS2.
At present, n-layer MoS2 can be grown by chemical vapor deposition (CVD)11–13 or be thinned to ultrathin flakes by the micromechanical cleavage method like that used for preparing graphenes on different substrates.1 The n-layer MoS2 prepared by the micromechanical cleavage method has the advantage of less defects and higher on/off ratios for MoS2 devices,7 indicating higher quality comparing to that grown by CVD. In this work, n-layer MoS2 were mechanically exfoliated from a piece of natural crystalline MoS2 sample (SPI Supplies) with Scotch transparent tape 600 (3M). Then the MoS2 were transferred to 300 nm SiO2/Si substrates by adhering and taking off the tape. The position and layers number were determined by optical microscope (Leica DM4000) and micro-Raman spectroscope (Renishaw in Via Raman Spectroscope). A film of Pd was evaporated onto n-layer MoS2. Before and after annealing treatment, scanning electron microscope (SEM) and micro-Raman spectroscope are used to study the morphologies and properties of Pd on n-layer MoS2. An interesting result is that by controlling the thickness of Pd film and annealing temperature, one or two top layers of n-layer MoS2 can be removed during the annealing process. The corresponding mechanism was proposed and discussed.
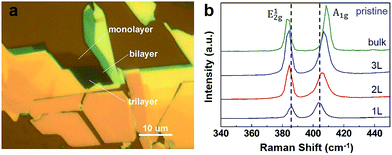
The n-layer MoS2 were first selected in an optical microscope based on their colour contrast as shown in Fig. 1a. The layer number is estimated according to the color contrast. And their coordinates were recorded according to a mark in these optical images. Then the Raman spectra of these n-layer MoS2 were measured, which has proved to be a powerful technology to identify the properties of ultrathin MoS2 flakes,14,15 especially the determination of the layer number of n-layer MoS2. The layer identification was based on the difference of Raman spectra among these n-layer MoS2.14 Raman spectra of n-layer MoS2 excited by 514 nm laser lines are measured as shown in Fig. 1b; thus, the layer number of n-layer MoS2 is determined accurately. More details of the experiment are given in the ESI.†
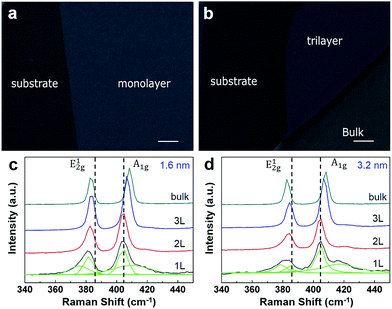
A Pd film with controllable thickness was evaporated onto MoS2 layers via a thermal evaporator at vacuum of 10−4 Pa. The morphologies of the Pd film were studied in detail by SEM. As shown in Fig. 2a and b, after thermal deposition of a Pd (1.6 nm) onto n-layer MoS2, the Pd forms a uniform films on the surface of MoS2 and substrate. The morphologies of Pd on the surface of MoS2 and substrate are different and the boundary between the substrate and n-layer MoS2 can be seen as shown in Fig. 2. Meanwhile, the morphologies of Pd on n-layer MoS2 with different layer numbers, such as n = 1, 3 and bulk, are not so clear as shown in Fig. 2a and b.
After deposition of Pd film on n-layer MoS2, their typical Raman spectra are shown in Fig. 2c and d for film thickness of 1.6 nm and 3.2 nm, respectively. For pristine n-layer MoS2, the two peaks at 384 cm−1 and 404 cm−1 which are ascribed to be E12g and A1g for laser line of 514 nm excitation. The E12g mode is in-plane which results from opposite vibration of the two S atoms with respect to the Mo atom while the A1g mode is associated with the out-of-plane vibration of only S atoms in opposite directions.16–18 These two Raman modes, E12g and A1g, exhibited sensitive to the thickness of MoS2. Because of the coulombic interactions and possible stacking-induced changes of the intra-layer bonding, the frequency of the E12g peak decreases and that of the A1g peak increases with increasing layer number of n-layer MoS2 as shown in Fig. 1b.
Comparing to the Raman spectra of pristine MoS2, the Raman spectra of n-layer MoS2 decorated Pd film have several significant changes. Firstly, as shown in Fig. 2c, the frequencies of E12g peak of Pd decorated n-layer MoS2 show obvious red shifts. And the shift increases with the increasing of the layer number of n-layer MoS2, which means that the Raman frequency shift of E12g peak is dependent on the layer number “n” of n-layer MoS2. For monolayer MoS2, the E12g peak has the largest frequency shift 4.0 cm−1. For bulk MoS2, the frequency shift of E12g peak almost disappears. Secondly, the A1g peak frequency of Pd decorated n-layer MoS2 shows similar increasing tendency as that in pristine MoS2 and the magnitudes of peak shifts of the A1g peaks are reduced obviously. For Pd decorated monolayer MoS2, the new shoulder peaks of the A1g peak and E12g peak can be found in the spectra. Thirdly, the intensity ratios of the Raman peaks (E12g and A1g) of Pd decorated n-layer MoS2 are different from those of pristine n-layer MoS2. The deposition of Pd film change the intensity ratios of E12g and A1g peaks, especially for bilayer and trilayer MoS2. For example, for pristine bilayer MoS2, the intensity of E12g peak is larger than that of A1g peak. But for Pd decorated bilayer MoS2, the intensity of E12g peak is weaker than that of A1g peak.
Meanwhile, it should be noted that the thickness of Pd has little effect on the modification of the Raman spectra of n-layer MoS2. As shown in Fig. 2d, the Raman spectra of bilayer and trilayer MoS2 decorated with 3.2 nm Pd film are almost the same as those of 1.6 nm Pd film decorated bilayer and trilayer MoS2. For monolayer MoS2, the Raman spectra of 3.2 nm Pd film decorated MoS2 show slight differences in the intensity of the characteristic peaks as shown the green component peaks in Fig. 2c and d.
An important and interesting question here is why Raman spectra of n-layer MoS2 changes after the deposition of Pd film. The changes of Raman spectra for metals on graphenes19–21 have been attributed to two main reasons: one is the doping effect, another is the stress effect. The changes of Raman spectra of n-layer MoS2 after the deposition of Pd film in this work are believed to the lattice mismatch between MoS2 and Pd, which is supported by the experimental result (the result will be shown in Fig. 3) that the changes of Raman spectra disappears after annealing.
In Fig. 3a and b, typical SEM images of the Pd on n-layer MoS2 after annealing at 800 °C for 4 min are shown. It can be seen that after annealing treatment, the Pd film has become nanoparticles on the substrate. On n-layer MoS2, the morphologies of Pd metal are quite different from those on the substrate. On monolayer and trilayer MoS2, the metal Pd has aggregated into irregular nanostrips. At the boundary between monolayer and trilayer MoS2, there are no Pd as shown in Fig. 3a. The difference of the morphologies of Pd on monolayer and trilayer MoS2 is small. For example, as shown in the Fig. 3a, the size of Pd strip on trilayer MoS2 is little larger than that on monolayer MoS2. And the size of the Pd nanostrips on bulk MoS2 is larger than those on monolayer and trilayer MoS2 as can be seen from Fig. 3b, which results in a low density on bulk MoS2. Therefore, it can be concluded that the size of the nanostrips becomes larger and the density becomes smaller with the increase of MoS2 layer number.
Why the Pd on n-layer MoS2 show the thickness-dependent morphologies? Thickness-dependent morphologies of metals on graphenes have been reported and the mechanism is attributed to thermodynamic (e.g., energetics and stability) and kinetic (e.g., surface diffusion) factors.22,23 Since similar systems (Pd on n-layer MoS2 versus metals on n-layer graphenes) are studied, the same mechanism is believed to be applicable. This is to say, the density of nanostrips (N) can be obtained using the following equation: N ∝ exp(En/3KT), where En is the diffusion barriers of Pd on n-layer MoS2, K is the Boltzmann constant, T is the temperature. Therefore, the different diffusion barrier En of Pd on substrate and n-layer MoS2 lead to the different nanostrips (N) and the thickness-dependent morphologies on Pd on n-layer MoS2.
The Raman spectra of Pd nanostrips on n-layer MoS2 have been measured. Typical Raman spectra of Pd nanostrips on n-layer MoS2 are shown in Fig. 3c after the Pd films of 1.6 nm on n-layer MoS2 have been treated at 750 °C for 4 minutes in vacuum. Comparing to those of pristine and Pd film coated n-layer MoS2, the Raman spectra after annealing treatments show interesting characteristics: firstly, the red shift of E12g peak in monolayer, bilayer MoS2 and its layer dependence in Pd film coated n-layer MoS2 almost disappear. Secondly, the positions of E12g peak and its layer dependence are quite similar to that of pristine n-layer MoS2 as shown in Fig. 3d, which is red-shift behaviour with the increasing layer. Thirdly, the position of the A1g peak do not change much among those of pristine, Pd decorated and heating treated n-layer MoS2. Fourthly, the intensity ratios between A1g and E12g after annealing have similar values as those of pristine n-layer MoS2.
The above results indicate that after annealing at 750 °C, the Raman spectra of 1.6 nm Pd film decorated n-layer MoS2 are quite similar to those of the pristine n-layer MoS2, suggesting the recovery of n-layer MoS2. In order to study the effect of the thickness of Pd metal, the thickness of Pd film is increased to 3.2 nm. As shown in Fig. 2c and d, the Raman spectra of n-layer MoS2 decorated with Pd films of 1.6 or 3.2 nm are almost the same, suggesting that the thickness of Pd films has little effect on the Raman spectra of Pd decorated MoS2.
After annealing, quite interesting and unexpected phenomena are observed and the results are shown in Fig. 3e. It shows the Raman spectra of the 3.2 nm Pd film decorated MoS2 after 750 °C annealing treatment in vacuum for 4 minutes. It can be seen that after annealing treatment, there is no any peaks observed for monolayer MoS2, indicating that the original monolayer MoS2 has disappeared. The optical images and Raman mapping of the monolayer MoS2 have been added as Fig. S4 in the ESI† to show the uniformity of the thinning behavior. The Raman spectrum of bilayer MoS2 has similar characteristics as that of the Raman spectrum of pristine monolayer MoS2 as shown in Fig. 1b and 3e. Meanwhile, the frequency difference of A1g and E12g is 20.2 cm−1 in bilayer MoS2, which is consistent with that of 19.5 cm−1 in pristine monolayer MoS2 (see Fig. 3f). For original trilayer MoS2, its Raman spectrum is similar to that of the Raman spectrum of pristine bilayer MoS2 (see Fig. 3e). And the frequency difference between A1g and E12g of 22.2 cm−1 is consistent with that of 21.7 cm−1 in pristine bilayer MoS2 (see Fig. 3f). These results indicate that for Pd film of 3.2 nm the top layer of n-layer MoS2 is removed after annealing at 750 °C in vacuum. Thus, after this treatment monolayer MoS2 disappears, bilayer MoS2 becomes monolayer MoS2 and so on. It should be noted that when few-layer MoS2 flakes are annealed in vacuum as long as one hour, one layer of MoS2 can be removed.24 If the vacuum annealing time is shorted to several minutes (Fig. S3 in ESI†), no removing behavior is observed, suggesting the important role of palladium film in the thinning behavior of n-layer MoS2.
These data suggest that the removal of the top layer of n-layer MoS2 depends on both the thickness of Pd metal and the temperature. For example, when the annealing temperature is increased to 800 °C for Pd film of 3.2 nm, both monolayer and bilayer MoS2 disappeared after annealing treatment as shown in Fig. 3f. The original trilayer MoS2 becomes a monolayer MoS2 after the annealing treatment. The frequency difference of A1g and E12g is 20.4 cm−1 in the original trilayer MoS2 as shown in Fig. 3f. These results indicate that the top two layers of the MoS2 can be successfully removed at the same time. This means that, if we control the annealing temperature and the thickness of Pd film, the number of layers removed can also be controlled.
The effects of annealing temperature and thickness of Pd on the removal of n-layer MoS2 are systematically studied and the results are summarized in Table 1. It can be seen that the thickness of Pd film plays an important role for the removal of the top layers of n-layer MoS2 after vacuum annealing. When the thickness of Pd is 1.3, 1.6 or 2.7 nm, no removal of the top layers of n-layer MoS2 is observed for annealing temperature of 750 °C/800 °C. When the thickness of Pd is 3.2 nm, one layer at the top of n-layer MoS2 is removed at annealing temperature of 750 °C. If the annealing temperature is raised to 800 °C, two layers at the top of n-layer MoS2 are removed. This means that after annealing monolayer and bilayer MoS2 disappear and trilayer MoS2 becomes a monolayer MoS2. Meanwhile, the photoluminescence (PL) spectra of these n-layer MoS2 were measured. The conclusion is shown in Fig. S1 and S2 (ESI†) which consistent with the result of Raman spectra. Since the thinning occurs only at the annealing process, the mechanism of thinning of n-layer MoS2 is attributed to a dissolving-like process of MoS2 in the metal Pd, which can be expressed by the following reaction:
| Thickness of Pd film | Annealing temperature | Results |
|---|---|---|
| 3.2 nm | 800 °C | 1 & 2L removed, 3L → 1L |
| 3.2 nm | 750 °C | 1L removed, 2L → 1L; 3L → 2L |
| 2.7 nm | 750 °C/800 °C | No removal |
| 1.6 nm | 750 °C/800 °C | No removal |
| 1.3 nm | 750 °C/800 °C | No removal |
Conclusions
In summary, when Pd is thermally evaporated onto n-layer MoS2, a uniform film is formed on the surface. If Pd/n-layer MoS2 is annealed at high temperature in vacuum, Pd nanostrips are observed on n-layer MoS2. The size of Pd nanostrips becomes larger and the density becomes smaller with the increase of layer number of n-layer MoS2, respectively. By studying the Raman and PL spectra, the thinning behavior of n-layer MoS2 has been observed. By controlling the thickness of Pd film and annealing temperature, one or two top layers of n-layer MoS2 can be removed during the annealing process. The mechanism of the thinning behavior is proposed and the thinning method presented in this work benefits the future design and fabrication of MoS2-based device.Acknowledgements
We thank the financial support from National Science Foundation of China (Grant No. 11174062, 51472057). W. G. Chu acknowledges financial support from the Strategic Priority Research Program of the Chinese Academy of Sciences (GrantXDA09040101).Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- K. Novoselov, A. K. Geim, S. Morozov, D. Jiang, M. Katsnelson, I. Grigorieva, S. Dubonos and A. Firsov, Nature, 2005, 438, 197 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530 CrossRef CAS PubMed.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699 CrossRef CAS PubMed.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 474 CrossRef PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147 CrossRef CAS PubMed.
- I. Song, C. Park and H. C. Choi, RSC Adv., 2015, 5, 7495 RSC.
- H. Li, Z. Y. Yin, Q. Y. He, H. Li, X. Huang, G. Lu, D. W. H. Fam, A. I. Y. Tok, Q. Zhang and H. Zhang, Small, 2012, 8, 63 CrossRef CAS PubMed.
- X. Zhao, P. Chen, C. Xia, T. Wang and X. Dai, RSC Adv., 2016, 6, 16772 RSC.
- Y. H. Lee, X. Q. Zhang, W. Zhang, M. T. Chang, C. T. Lin, K. D. Chang, Y. C. Yu, J. T. W. Wang, C. S. Chang and L. J. Li, Adv. Mater., 2012, 24, 2320 CrossRef CAS PubMed.
- Y. Zhan, Z. Liu, S. Najmaei, P. M. Ajayan and J. Lou, Small, 2012, 8, 966 CrossRef CAS PubMed.
- Z. Lin, Y. Zhao, C. Zhou, R. Zhong, X. Wang, Y. H. Tsang and Y. Chai, Sci. Rep., 2015, 5, 18596 CrossRef CAS PubMed.
- H. Li, Q. Zhang, C. C. R. Yap, B. K. Tay, T. H. T. Edwin, A. Olivier and D. Baillargeat, Adv. Funct. Mater., 2012, 22, 1385 CrossRef CAS.
- S.-L. Li, H. Miyazaki, H. Song, H. Kuramochi, S. Nakaharai and K. Tsukagoshi, ACS Nano, 2012, 6, 7381 CrossRef PubMed.
- P. A. Bertrand, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 5745 CrossRef CAS.
- D. Bae, K. Kim, Y. Park, E. Suh, K. An, J.-M. Moon, S. Lim, S. Park, Y. Jeong and Y. Lee, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 233401 CrossRef.
- C. Lee, H. Yan, L. E. Brus, T. F. Heinz, J. Hone and S. Ryu, ACS Nano, 2010, 4, 2695 CrossRef CAS PubMed.
- A. Das, S. Pisana, B. Chakraborty, S. Piscanec, S. Saha, U. Waghmare, K. Novoselov, H. Krishnamurthy, A. Geim and A. Ferrari, Nat. Nanotechnol., 2008, 3, 210 CrossRef CAS PubMed.
- R. Muszynski, B. Seger and P. V. Kamat, J. Phys. Chem. C, 2008, 112, 5263 CAS.
- Z. H. Ni, T. Yu, Y. H. Lu, Y. Y. Wang, Y. P. Feng and Z. X. Shen, ACS Nano, 2008, 2, 2301 CrossRef CAS PubMed.
- Y. W. Mo, J. Kleiner, M. B. Webb and M. G. Lagally, Phys. Rev. Lett., 1991, 66, 1998 CrossRef PubMed.
- L. Y. Ma, L. Tang, Z.-L. Guan, K. He, K. An, X.-C. Ma, J.-F. Jia, Q. K. Xue, Y. Han, S. Huang and F. Liu, Phys. Rev. Lett., 2006, 97, 266102 CrossRef PubMed.
- X. Lu, M. I. B. Utama, J. Zhang, Y. Zhao and Q. Xiong, Nanoscale, 2013, 5, 8904–8908 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental conditions of Raman measurements and other data. See DOI: 10.1039/c6ra07714e |
| This journal is © The Royal Society of Chemistry 2016 |