Reduced graphene oxide-stabilized copper nanocrystals with enhanced catalytic activity and SERS properties†
Meizhen Guo,
Youcheng Zhao,
Fengying Zhang,
Li Xu,
Hongfang Yang,
Xinyu Song* and
Yuxiang Bu
School of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, P. R. China. E-mail: songxy@sdu.edu.cn
First published on 19th May 2016
Abstract
Well-defined Cu/reduced graphene oxide (rGO) hybrid materials are successfully synthesized by controlling the amount of ascorbic acid and maintaining an appropriate pH value. We found that graphene oxide (GO) served not only as the precursor for graphene, but also as an effective surfactant to hamper the aggregation of copper nanoparticles, resulting in a small size of the copper nanoparticles. Furthermore, the as-prepared copper composites can serve as an effective catalyst for 4-nitrophenol in aqueous conditions and exhibit surface enhanced Raman scattering in the detection of crystal violet (CV). Notably, the obtained copper nanoparticle hybrids with rGO have extremely high air stability after exposure to air. Density functional theory calculations firstly reveal that rGO can effectively prevent Cu nanoparticles from spontaneous oxidation due to its slightly lower ionization potential than that of Cu nanoparticles. We expect the as-prepared rGO-stabilized copper nanocrystals with small size to meet the increasing demands of industrial applications at reduced costs.
1. Introduction
Metal (Au, Ag, Pt, Pd and Cu) nanoparticles and nanostructures have become of great interest in recent years owing to the versatility of their applications in the fields of catalysis, optics, biochemical sensing, electronics and spectroscopy.1–6 In comparison with Ag or Au, copper is a cost-effective and desirable alternative because of its unique physical and chemical properties and widespread range of potential applications in nanoscience.7 However, the issues of copper nanoparticles such as the stabilization of particles against agglomeration, the achievement of monodisperse size distributions and oxidative stability have presented considerable obstacles for their practical application. Especially, the susceptibility of copper nanoparticles to oxidation is enhanced when the particle size is reduced, which degrades their desired properties and limits their potential applications. To fully utilize the properties of copper nanoparticles, the chemical stability must be well controlled.8 So far, there are a few reports on the synthesis of air-stable and thermally stable copper nanoparticles.9–14 For example, Qi et al. developed a grafted method to assemble and grow epitaxially copper ions in situ on a Si wafer. The Si wafer renders copper nanoparticles with a mean size distribution of 20 nm and they exhibit stability.9 M. Ibrahim Dar et al. developed a microwave-assisted method to prepare air-stable copper nanostructures.12In recent years, Cu nanoparticles coated with carbon, have attracted intense interest, because the carbon shells can serve as shields to protect the Cu nanocores from oxidation.15–19 To date, various preparation methods of copper–carbon composites have been described extensively, such as arc discharge in different atmospheres,11,17 microwave irradiation,18,19 chemical vapor deposition15 and thermolysis of organometallic compounds.10,16 However, as general carbon shells are usually amorphous or too thick, chemical sites of the metallic (core) are always retarded.10,11 As a result, the new carbon materials with unique architectures forms are desirable to be developed for preparing the copper–carbon composites with both oxidation stability and unhindered chemical reactivity.
It is known that graphene, a novel thin carbon material with unique structure and properties, can serve as molecular templates hybridizing with metal nanoparticles to fabricate graphene-based composites.20 To date, there are some reports of the synthesis of graphene–copper hybrids.15,21–23 In general, the various methods of obtaining graphene–coated copper nanomaterials require rigorous condition such as high temperature and energy consumption, which is the obstacle to the practical application. For example, in the articles of Luechinger et al. and Athanassiou et al., Cu nanoparticles with enhanced thermal stability and electrical conductivity were obtained after capping with few layers of graphene using flame synthesis.21,22 Taeyoon et al. reported the encapsulation of spherical Cu nanoparticles with few layers of graphene using a solid phase carbon source of poly(methyl methacrylate) (PMMA) during a CVD process at a high temperature (800–900 °C).23 Recently, several groups have reported the synthesis of copper–graphene hydrids using the conventional chemical solution methods.24–26 For example, in ref. 24, Cu/rGO composites were prepared using relatively expensive NaBH4 at 100 °C for 24 h. In a recent report of Nasrollahzadeh, Cu/rGO composites were prepared by mixing pre-prepared copper nanoparticles and rGO power at 110 °C for 12 h. Now, it is still highly required to develop facile and environmental-friendly chemical solution methods for the preparation of Cu/grapheme materials.
In this work, a facile wet-solution approach for the synthesis of copper nanoparticles supported on the reduced graphene oxide is demonstrated by simultaneously reducing graphene oxide (GO) and copper(II) salt using L-ascorbic acid as the reducing agent under atmosphere air conditions for 1 h. With a moderate amount of L-ascorbic acid and the optimal pH value, the well distributed copper nanoparticles with a size ranging from 5 nm to 10 nm on the rGO sheets are obtained, in which rGO sheets might work as a planar substrate for preventing agglomeration of copper nanoparticles. Notably, the rGO-supported copper nanocrystals exhibit excellent oxidative resistance and keep non-oxidized for months in the ambient condition. The extremely high stability of such copper nanocrystals is firstly ascertained on the basis of the density functional theory calculations. It is worth mentioning that this Cu/rGO nanocomposites can enhance excellent catalytic activity for the reduction of 4-nitrophenol in comparison with pure bulk copper nanoparticles and exhibit surface enhanced Raman scattering in the detection of crystal violet.
2. Materials and methods
2.1 Materials
NaOH, CuCl2·2H2O H2SO4, KMnO4, H2O2 (30%), HCl, ascorbic acid, and ethanol were obtained from Chengdu Kelong Chemical Reagent Company. Graphite powder, 4-nitrophenol, and crystal violet were specpure grade and obtained from Aladdin.2.2 Typical preparation of nanostructures
2.3 Catalytic activity and SERS study
(2) 0.085 mg Cu/rGO composites were dispersed in 1.0 mL of 4-nitrophenol aqueous solution (0.3 mM) at room temperature. Then a freshly prepared aqueous solution of NaBH4 (1.0 mL, 0.02 M) was added. The mixture was immediately transferred into a quartz cuvette. The progress of the reactions was monitored with a UV vis-NIR spectrophotometer.
2.4 Structural/microstructural characterization
The samples were characterized by XRD on a German Bruker D8 X-ray diffractometer with Ni filtered Cu Kα radiation (λ = 1.5418 Å). Fourier transform infrared (FT-IR) spectra were recorded on a FTS 135 FT-IR spectrophotometer using the KBr pellet method. Raman spectroscopy was recorded on a Horiba LabRAM HR800 with excitation from the 514 nm line of an Ar-ion laser with a power of about 5 mW. The morphologies of the samples were examined by transmission electron microscopy (TEM JEM-100CXII). N2 adsorption–desorption was tested on Quantachrome in nitrogen atmosphere. Thermogravimetric analysis (TGA) was performed using SDT Q600. The samples were heated from room temperature to 800 °C at a rate of 10 °C min−1 in air atmosphere. X-ray photoelectron spectroscopy (XPS) spectrum were measured on ESCALAB 250 with a monochromatized Mg-Kα source and a resolution of 1.00 eV.3. Results and discussion
3.1 Phase structure, morphology, and formation process
L-Ascorbic acid is the only one reducing agent used in this reaction.The amount of L-ascorbic acid should be important for the component of the nanoparticles grown on graphene. Fig. 1 shows the X-ray diffraction (XRD) patterns of the as-prepared nanocomposites by varying the usage of L-ascorbic acid solution (0.25 M) at a fixed pH = 10, respectively. As shown in Fig. 1e, all the diffraction lines of the product are assigned to Cu on the basis of JCPDS 04-0836 card. That is to say only the amount of L-ascorbic acid (0.25 M) surpassed 3.0 mL, the Cu/rGO nanocomposites can be obtained. In addition, from the XRD patterns in Fig. 1a–d, it can be seen that the graphene based nanomaterials with the coexistence of copper and cuprous oxide are obtained between the amount of L-ascorbic acid of 2.0 mL and 2.8 mL. When 1.0 mL is used, we can obtain the graphene hybridized with cuprous oxide. When the amount of L-ascorbic acid (0.25 M) is 0.5 mL, only copper oxide/rGO can be obtained. It is observed that the diffraction peaks of the CuO/rGO nanocomposite are wider than that of other nanocomposites, probably demonstrating that the product has lower crystallinity. Besides, the peak belonging to graphite is not obvious in those composites, indicating the decoration of nanoparticles onto the rGO sheets caused the enlargement and disorder in the layer spacing of graphene. A HRTEM image taken from the selected area of the Cu/rGO composites is shown in Fig. S1.† The stacking width is calculated to be 0.34 nm for rGO layers corresponding to the characteristic inter-graphene spacing.29 The observed interplanar spacing of 0.21 nm corresponds to the (111) lattice plane of Cu,30 which further reveals that the formed Cu nanocrystals are deposited on rGO sheets.
FT-IR and Raman spectrum analyses of the as-prepared Cu/rGO nanocomposites comparing with other materials are also shown in Fig. 2. As illustrated in Fig. 2A(d), absorption bands of GO are observed at 1728, 1224, and 1053 cm−1, which are attributed to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O of epoxy and alkoxy, respectively. In addition, the peak at 1628 cm−1 is assigned to the contributions from the skeletal vibrations of the graphitic domains, and the peaks at 1622, 1384, and 1224 cm−1 correspond to the stretching vibration of carboxyl groups. Obviously, Fig. 2A(a and c) indicate that these peaks become weak or disappear when GO is reduced using L-AA. The FT-IR spectrum of the Cu/rGO nanocomposites in Fig. 2A(a) is similar to those of rGO (Fig. 2A(c)), implying that the number of hydroxyl, carbonyl, and carboxyl groups in the Cu/rGO nanocomposites substantially decrease after chemical reduction.31,32 Also, there is no observable absorption peak when the wavenumber is above 750 cm−1 for pure Cu nanoparticles (Fig. 2A(b)). Raman spectroscopy is known as a suitable technique to study the ordered/disordered crystal structures of carbonaceous materials. Fig. 2B shows the Raman spectrum of GO and the Cu/rGO composite, respectively. Two characteristic bands at about 1330 and 1594 cm−1 (where ID and IG are the D-peak and G-peak Raman intensities, respectively) are observed in Raman spectrum of graphene oxide and Cu/rGO composites, which is related to the vibration of the sp2-bonded carbon atoms in a 2-dimensional hexagonal lattice and the defects and disorder in the hexagonal graphitic layers. For the hybrid Cu/rGO, the intensity ratio (1.14) of the D-band to G-band is higher than that (0.89) for GO, indicating that more defects have been doped into the Cu/rGO and a decrease in the average size of the sp2 domains upon the efficient reduction of GO. X-Ray photoelectron spectroscopy (XPS) measurement was performed to further estimate the composition of the Cu/rGO hybrids, and the results are shown in Fig. S2.† The XPS spectrum of the C 1s of the rGO (Fig. S4a†) exhibits five peaks, and these peaks at 284.55 eV, 285.65 eV, 286.65 eV, 287.95 eV, 289.0 eV can be assigned to sp2C, sp3C, –C–O, –C
O, C–O of epoxy and alkoxy, respectively. In addition, the peak at 1628 cm−1 is assigned to the contributions from the skeletal vibrations of the graphitic domains, and the peaks at 1622, 1384, and 1224 cm−1 correspond to the stretching vibration of carboxyl groups. Obviously, Fig. 2A(a and c) indicate that these peaks become weak or disappear when GO is reduced using L-AA. The FT-IR spectrum of the Cu/rGO nanocomposites in Fig. 2A(a) is similar to those of rGO (Fig. 2A(c)), implying that the number of hydroxyl, carbonyl, and carboxyl groups in the Cu/rGO nanocomposites substantially decrease after chemical reduction.31,32 Also, there is no observable absorption peak when the wavenumber is above 750 cm−1 for pure Cu nanoparticles (Fig. 2A(b)). Raman spectroscopy is known as a suitable technique to study the ordered/disordered crystal structures of carbonaceous materials. Fig. 2B shows the Raman spectrum of GO and the Cu/rGO composite, respectively. Two characteristic bands at about 1330 and 1594 cm−1 (where ID and IG are the D-peak and G-peak Raman intensities, respectively) are observed in Raman spectrum of graphene oxide and Cu/rGO composites, which is related to the vibration of the sp2-bonded carbon atoms in a 2-dimensional hexagonal lattice and the defects and disorder in the hexagonal graphitic layers. For the hybrid Cu/rGO, the intensity ratio (1.14) of the D-band to G-band is higher than that (0.89) for GO, indicating that more defects have been doped into the Cu/rGO and a decrease in the average size of the sp2 domains upon the efficient reduction of GO. X-Ray photoelectron spectroscopy (XPS) measurement was performed to further estimate the composition of the Cu/rGO hybrids, and the results are shown in Fig. S2.† The XPS spectrum of the C 1s of the rGO (Fig. S4a†) exhibits five peaks, and these peaks at 284.55 eV, 285.65 eV, 286.65 eV, 287.95 eV, 289.0 eV can be assigned to sp2C, sp3C, –C–O, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –COO groups, respectively.33 It can be seen that the intensity of the sp3C is obvious weak compared with that of sp2C and the intensities of the containing oxygen groups are also small, which are in good agreement with the IR and Raman. In addition, Cu 2p3/2 and 2p1/2 peaks at 932.6 eV, 952.3 eV should correspond to the characteristic Cu0 peaks.34 Satellite peaks centered at 945 eV of Cu2O and CuO are absent, demonstrating the product has a zerovalence of metallic copper.34 This further confirms that the Cu/rGO nanocomposites have been successfully prepared via reduction of GO with L-AA.35 TGA is used to further investigate the content of copper in the as-prepared Cu/rGO nanocomposite. As shown in Fig. S3,† no significant mass loss is detected when the Cu/rGO nanocomposite are heated up to 500 °C. The content of Cu in the nanocomposite is about 81 wt% by thermogravimetric analysis (TGA), which is fairly close to the theoretical value (80.5 wt%) of the nanocomposites based on the experimental conditions (according to comparative experiments, 100 mg of GO could be reduced to about 50 mg of rGO).
O and –COO groups, respectively.33 It can be seen that the intensity of the sp3C is obvious weak compared with that of sp2C and the intensities of the containing oxygen groups are also small, which are in good agreement with the IR and Raman. In addition, Cu 2p3/2 and 2p1/2 peaks at 932.6 eV, 952.3 eV should correspond to the characteristic Cu0 peaks.34 Satellite peaks centered at 945 eV of Cu2O and CuO are absent, demonstrating the product has a zerovalence of metallic copper.34 This further confirms that the Cu/rGO nanocomposites have been successfully prepared via reduction of GO with L-AA.35 TGA is used to further investigate the content of copper in the as-prepared Cu/rGO nanocomposite. As shown in Fig. S3,† no significant mass loss is detected when the Cu/rGO nanocomposite are heated up to 500 °C. The content of Cu in the nanocomposite is about 81 wt% by thermogravimetric analysis (TGA), which is fairly close to the theoretical value (80.5 wt%) of the nanocomposites based on the experimental conditions (according to comparative experiments, 100 mg of GO could be reduced to about 50 mg of rGO).
 | ||
| Fig. 2 (A) FTIR spectra of (a) CuO/rGO nanocomposite, (b) pure Cu, (c) rGO, and (d) GO; (B) Raman spectra of (a) Cu/rGO nanocomposite, (b) GO, respectively. | ||
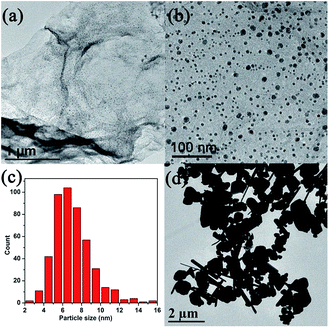
Furthermore, it is found that the morphology of the as-prepared Cu/rGO nanocomposites strongly depends on the pH value in this reaction. Keeping a fixed amount of L-ascorbic acid (3.0 mL), the morphologies of copper nanoparticles grown on the rGO nanosheets are studied. At pH = 6 and 8 as shown in Fig. 3a and b, non-uniform copper nanoparticles are observed on the rGO sheets with a typical rippled and crumpled structure. While further increasing the pH to 10 and 12, as indicated in Fig. 3c and d, the surfaces of rGO sheets are densely covered by narrowly distributed copper nanoparticles with a size about 5 to 10 nm. The decrease of particle size and increase of particle uniformity in the pH between 10 and 12 should be attributed to the elevated nucleation rate with the ascorbic acid reductant in higher alkaline pH. In addition, the relatively higher alkaline solution contributes to lowering nucleation energy and mediating the number of the nucleation sites on GO sheets significantly, and thus copper nanoparticles with smaller sizes can be obtained in the final Cu/rGO composite.36–38 Control experiments have also been taken to investigate the impact of GO on growth of copper nanocrystals. In the absence of GO, the pure Cu products are prepared at different pH = 6, 8, 10, 12, respectively. The corresponding TEM images are shown in Fig. S4† and 4d. It is evident that the as-obtained products show obvious aggregation and irregular morphology and large particle sizes (Fig. 4d and S4†). Obviously, it differs from the cases of copper growth on GO sheets. Fig. 4c shows the size distribution of Cu nanoparticles in Cu/rGO composites (pH = 10) based on TEM image. It clearly shows that the range of size distribution is wide from 2 to 20 nm and concentrates on 5–10 nm, matching well with the results of the magnified TEM image characterization in Fig. 4b. The SEM image of the products formed at pH = 10 in Fig. S5† further demonstrates that Cu nanoparticles are homogeneously distributed on the surface of rGO without aggregation. Clearly, GO could serve as an effective surfactant to inhibit aggregation of copper nanoparticles, resulting in a small size of the copper nanoparticles. So we choose the optimal pH value at 10 for further research.
 | ||
| Fig. 3 TEM images of Cu/rGO nanocomposite prepared with different pH values. (a) pH = 6, (b) pH = 8, (c) pH = 10 and (d) pH = 12. | ||
On the basis of the above results, the possible formation process of the Cu/rGO nanocomposites is illustrated in Fig. 5. Firstly, in the mixed aqueous of GO and copper(II) salts, some Cu2+ are attracted by negatively charged oxygen-containing groups on GO through electrostatic interaction and serve as nucleation sites. Secondly, Cu(OH)2 nanoparticles are achieved after the addition of the solution of NaOH. Thirdly, L-ascorbic acid as a reductant transforms Cu(OH)2 to Cu and simultaneously reduces GO to rGO sheets under the heat treatment condition. The GO sheets could play a role of analogous surfactant in growing Cu nanocrystals, eliminating aggregation and improving stability. Meanwhile, the decorated Cu nanocrystals as spacer also help to prevent the aggregation of rGO.39,40 In order to further clarify the synthesis mechanism, the XRD and TEM of the formed intermediate samples before adding ascorbic acid are given in Fig. S6.† The XRD and TEM results clearly indicates Cu(OH)2 are firstly formed on the rGO in the second step.
The oxidative stability of the as-prepared samples is examined by using the XRD pattern. As can be seen from Fig. 6a, the XRD pattern of the as-prepared pure Cu nanoparticles in air for 3 months shows that the impurity peaks belong to Cu2O (Fig. 6a). Whereas, the as-prepared Cu/rGO nanocomposites present greater air stability and chemical inertness after being stored for 3 months under the ambient conditions, and no impurity peaks appear (Fig. 6b). These observations reflect that the Cu/rGO nanocomposites exhibit excellent air stability.41 This excellent air stability could be ascribed to the protecting role of the rGO sheets, which is believed to be able to serve as shields to prevent the spontaneous oxidation of Cu nanoparticles in air.
 | ||
| Fig. 6 XRD patterns of (a) Cu/rGO nanocomposite and (b) pure Cu nanoparticles after exposed to ambient atmosphere for 3 months. | ||
To further unveil the origin of the observed excellent air-stability of such Cu/rGO nanocomposites, the density functional theory calculations were carried out. The B3LYP exchange-correlation functionals42–44 and a hybrid basis set (a 6-31G* basis set for C, O, and H and a LanL2DZ basis set for Cu) were used in the calculations. Since it is very difficult and even impossible to determine the actual structures of possible rGO patches and Cu/rGO nanocomposites, three arbitrarily modeled rGO molecular patches and a Cu/rGO cluster were also considered, as shown in Fig. 7. The former two correspond to the partially reduced GO model patches, while the last one corresponds to a rGO model. Their vertical and adiabatic ionization potentials are calculated to be 6.409/6.332 eV, 6.382/6.296 eV, and 5.902/5.802 eV, respectively. Although these ionization potentials of the partially oxidized GO models are larger than that of the rGO model, they are considerably smaller than the first ionization potential (7.726 eV) of Cu. This observation clearly indicates that this kind of rGO patches are more easily oxidized than the Cu clusters. To further clarify the relative oxidizability of the two moieties in the Cu/rGO hybrids, five different contact modes for the model hybrids are considered, as shown in Fig. 7B. No matter for the edge-interacting Cu/rGO (first) or for the surface contact Cu/rGO with different contact modes, the ionization-formed hole or spin density distributions mainly are at the rGO patch moieties (Fig. 7B). Clearly, these observations further indicate that the rGO patches lose electrons instead of the Cu cluster moiety upon oxidization or ionization and thus could protect the formed Cu nanoparticles from oxidization in their Cu/rGO hydrides. The contact mode between the Cu cluster and the patch moiety hardly affects their relative oxidizability. Of course, this is only a simple confirmation based on the electronic structure calculations for the observed phenomena, and further calculations targeting large Cu/rGO hybrid molecular cluster models are needed for detailed analyses.
3.2 Catalytic properties
The reduction of 4-nitrophenol by sodium or potassium borohydride has been used extensively as a model reaction to evaluate the catalytic activities of metal nanoparticles or hybrids.40,45–49 In this study, the reduction of 4-nitrophenol into 4-aminophonol by NaBH4 is employed as a model reaction to evaluate the enhanced catalytic activity of the as-synthesized Cu/rGO nanocomposites. Fig. 8A shows the successive UV-visible absorption spectra for the mixture of 10 mL 1 mM aqueous solution of 4-nitrophenol and 10 mL 10 mM aqueous solution of sodium borohydride (NaBH4) without any catalyst. It was observed that the peak at 415 nm UV-visible absorption spectra hardly changes for an hour, which proves that the NaBH4 is incapable of reducing in the absence of catalyst. Interestingly, while the addition of a fixed amount Cu/rGO nanocomposite (5 mg) or pure copper (5 mg) catalysts to the aqueous solution in the same condition, we found the obvious changes in the peak at 415 nm UV-visible absorption spectra. As shown in Fig. 8B, in the presence of the as-prepared bare Cu catalysts, reducing 4-nitrophenol to 4-aminophonol by NaBH4 completely was observed within 30 min. While used the as-prepared Cu/rGO nanocomposite catalysts, reducing 4-nitrophenol to 4-aminophonol by NaBH4 completely was observed within 15 min under the same condition. Fig. 8D displays the linear relationship of depletion of the 400 nm peak by measuring UV-vis absorption recorded at different times (i.e., ln(A) vs. reduction time), suggesting that the reaction is a pseudo-first-order. Obviously, the ranking procedure of the rate constants estimated from the plots is Cu/rGO nanocomposite (0.19 min−1) > pure copper nanoparticles (0.11 min−1). In addition, the catalytic activities of the Cu/rGO composites synthesized at pH = 6, 8, 12, are also examined (Fig. S7†), respectively. It can be seen that the order of catalytic activities towards the reduction of 4-nitrophenol is as follows: Cu/rGO (at pH = 10) ≈ Cu/rGO (at pH = 12) > Cu/rGO (pH = 6) ≈ Cu/rGO (pH = 8) > bare Cu. For further understanding the excellent catalytic activity of the as-prepared Cu/rGO nanocomposite, the BET surface area of the as-synthesized Cu/rGO nanocomposite at pH = 6, 8, 10, 12 are measured, and the corresponding parameters including the BET surface areas and the pore size are listed in Table S1,† respectively. The isotherm curves for all samples display a hysteresis loop, indicating the presence of large mesopores and macropores, which can be categorized as type IV according to IUPAC classification (shown in Fig. S8†). As shown in Fig. 9, the samples obtained at pH = 10 has a high BET surface of 40.7 m2 g−1, and an average pore width of 4.123 nm. A further observation shows that the BET surface areas of the as-prepared Cu/rGO at pH = 10 (40.7 m2 g−1) and the Cu/rGO obtained at pH = 12 (40.1 m2 g−1) are almost same. The same situation occurs for the formed Cu/rGO at pH = 6 and Cu/rGO at pH = 8. Moreover, the BET surface areas of Cu/rGO (pH = 10) and Cu/rGO (pH = 12) are higher than that of products obtained at pH = 6 or 8. It is also found that the pore volume of the as-obtained Cu/rGO products at pH = 10 or 12 shows a slight increase compared with that of Cu/rGO obtained at pH = 6 (or pH = 8). In addition, it is observed that the BET surface area of the as-prepared Cu/rGO nanocomposite is larger than that of the bare copper nanoparticles (20 m2 g−1, see Fig. S9†). It is well known that the lager BET surface areas, small sizes, and good dispersion state are helpful to improve the catalytic activity. Thus, the as-synthesized Cu/rGO nanocomposites at pH = 10 (or 12) act more active catalyst than pure Cu catalyst and other Cu/rGO composites. | ||
| Fig. 8 Successive UV-Vis spectra of the reduction reaction. (A) In the absence of any catalyst, (B) pure copper nanoparticles, (C) Cu/rGO nanocomposite. (D) Absorbance ln(A) versus time plot for the reduction of 4-NP in different conditions (corresponding to Fig. 6 A–C). | ||
 | ||
| Fig. 9 (a) N2 adsorption–desorption isotherm of the Cu/rGO nanocomposite and (b) BJH pore size distribution. | ||
In addition, the reduction reaction catalyzed by the as-prepared Cu/rGO nanocomposite was tested again with another method described in the Experimental section 2.3 (2), for the purpose of comparing our result with some noble catalysts as reported for a meaningful situation. The activity factor (K) was defined as the rate constant (k) from the slope over the total weight of the catalyst.45 By analyzing the absorption spectra of the reaction solution and absorbance ln(A) vs. time plot of the Cu/rGO nanocomposite as shown in Fig. S10 (ESI†), the K value was estimated to be 0.045 s−1 mg−1, which was higher than the Au/grapheme hydrogel,50 Ag–Au Nanowires.51 The data revealed that the as-prepared Cu/rGO nanocomposite shows more enhanced performance in a catalytic aspect than many previously reported noble catalysts in Table 1.52,53
| Samples | Quality/mg | Rate constant (k)/s−1 | Activity factor (K)/s−1 mg−1 | Reference |
|---|---|---|---|---|
| Cu/rGO | 0.085 | 0.0038 | 0.045 | This work |
| Ag/RGO | 0.1 | 0.0036 | 0.0036 | 52 |
| Au/grapheme hydrogel | 0.1 | 0.0031 | 0.031 | 50 |
| Ag–Au nanowires (1%) | 0.0625 | 0.0014 | 0.022 | 51 |
| Pure Ag nanowires | 0.0625 | 0.0007 | 0.0112 | 51 |
| Dendritic Ag/Au | 0.6 | 0.0061 | 0.0101 | 53 |
3.3 SERS study
Surface-enhanced Raman scattering (SERS), a powerful analytic technology, has been mainly existence in the coinage metals (Au, Ag, and Cu). Compared to noble metals Au and Ag, Cu substrates have been thought to have less significant SERS effects.9,54 However, in our work, With the use of graphene, there is no need to employ a capping agent. The rGO-stabilized Cu nanocrystals can overcome its poor oxidative stability. Moreover, Control of metal nanoparticle size and loading on graphene sheets is an important factor for applications such as SERS sensing.55 So the rGO-stabilized Cu nanocrystals might be good substrates for studying the SERS activity of the products. Raman spectra were collected, employing crystal violet (CV) as the probe molecule. As shown in Fig. 10, the SERS spectra obtained from the pure copper nanoparticles and the rGO-stabilized Cu nanocrystals absorbed with crystal violet (0.01 mM) solution are well-presented. In comparison with the Raman scattering spectra of CV (0.01 mM) solution with pure Cu nanoparticles, it is clear that CV on the SERS-active substrates of the rGO-stabilized Cu nanocrystals has large enhancements at 1619, 1522, 1178, 805, and 610 cm−1, indicating the potential of the sensing of probe molecules. This good performance is linked to the high dispersion of Cu species on the graphene surface in the reduced state.56 In addition, the presence of a graphene-based phase in these materials helped with separation of electrons and holes, preventing their recombination, and contributed to charge transfer. Of course, it is needed to further study for detecting the crystal violet with lower concentration for practical application.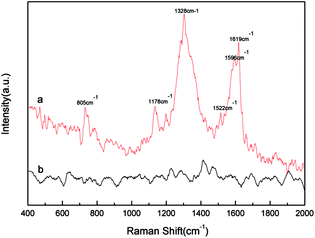 | ||
| Fig. 10 Raman spectrum of 10−5 M crystal violet (CV) solution adsorbed on the graphene-stabilized Cu nanocrystals and pure copper nanoparticles, respectively. | ||
4. Conclusions
In summary, we successfully achieved a simple wet-solution route for preparing rGO-stabilized Cu nanocrystals utilizing the GO as a special ‘‘surfactant’’ to tailor the size and morphology of the Cu nanocrystals. Experimental results and theoretical analysis indicate that rGO sheets in the Cu/rGO nanocomposites can serve as a shield to hinder the oxidation of the Cu nanoparticles. Compared to pure Cu nanoparticles, the rGO-stabilized Cu nanocrystals possess a higher catalytic efficiency to the reduction of p-nitrophenol into p-aminophenol at room temperature, which links its larger specific surface area and the porous structure and small particle sizes. Furthermore, the as-prepared Cu/rGO composites have a better SERS property than that of Cu nanoparticles. Our investigation likely initiates a handy way for the large-scale synthesis of the well-defined and air-stable graphene nanostructures with more application possibilities.Acknowledgements
This work was supported by the ZR2013BM027.Notes and references
- J. A. Fan, C. Wu, K. Bao, J. Bao, R. Bardhan, N. J. Halas, V. N. Manoharan, P. Nordlander, G. Shvets and F. Capasso, Science, 2010, 328, 1135 CrossRef CAS PubMed.
- P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 41, 1578 CrossRef CAS PubMed.
- M. Rycenga, C. M. Cobley, J. Zeng, W. Li, C. H. Moran, Q. Zhang, D. Qin and Y. Xia, Chem. Rev., 2011, 111, 3669 CrossRef CAS PubMed.
- S. Saha, A. Pal, S. Kundu, S. Basu and T. Pal, Langmuir, 2009, 26, 2885 CrossRef PubMed.
- P. Zhang, J. He, X. Ma, J. Gong and Z. Nie, Chem. Commun., 2013, 49, 987 RSC.
- N. Kränzlin, S. Ellenbroek, D. Durán-Martín and M. Niederberger, Angew. Chem., Int. Ed., 2012, 51, 4743 CrossRef PubMed.
- P. Lignier, R. Bellabarba and R. P. Tooze, Chem. Soc. Rev., 2012, 41, 1708 RSC.
- P. Kanninen, C. Johans, J. Merta and K. Kontturi, J. Colloid Interface Sci., 2008, 318, 88 CrossRef CAS PubMed.
- Q. Shao, R. Que, M. Shao, L. Cheng and S. T. Lee, Adv. Funct. Mater., 2012, 22, 2067 CrossRef CAS.
- H. Li, W. Kang, B. Xi, Y. Yan, H. Bi, Y. Zhu and Y. Qian, Carbon, 2010, 48, 464 CrossRef CAS.
- B. Bokhonov and S. Novopashin, J. Nanopart. Res., 2010, 12, 2771 CrossRef CAS.
- M. I. Dar and S. A. Hivashankar, J. Mater. Chem., 2012, 22, 22418 RSC.
- V. Engels, F. Benaskar, D. A. Jefferson, B. F. G. Johnson and A. E. H. Wheatley, Dalton Trans., 2010, 39, 6496 CAS.
- Y. Kobayashi, S. Ishida, K. Ihara, Y. Yasuda, T. Morita and S. Yamada, Colloid Polym. Sci., 2009, 287, 877 CAS.
- S. Wang, X. Huang, Y. He, H. Huang, Y. Wu, L. Hou, X. Liu, T. Yang, J. Zou and B. Huang, Carbon, 2012, 50, 2119 CrossRef CAS.
- J. Li and C.-y. Liu, New J. Chem., 2009, 33, 1474 RSC.
- H. Lei, Y. Tang, J. Li, J. Luo and J. Zhang, Appl. Phys. Lett., 2007, 91, 113119 CrossRef.
- D. S. Jacob, I. Genish, L. Klein and A. Gedanken, J. Phys. Chem. B, 2006, 110, 17711 CrossRef CAS PubMed.
- Y. L. Hsin, C. F. Lin, Y. C. Liang, K. C. Hwang, J. C. Horng, J. a. A. Ho, C. C. Lin and J. R. Hwu, Adv. Funct. Mater., 2008, 18, 2048 CrossRef CAS.
- I. V. Lightcap, T. H. Kosel and P. V. Kamat, Nano Lett., 2010, 10, 577 CrossRef CAS PubMed.
- N. A. Luechinger, E. K. Athanassiou and W. J. Stark, Nanotechnology, 2008, 19, 445201 CrossRef PubMed.
- E. K. Athanassiou, R. N. Grass and W. J. Stark, Nanotechnology, 2006, 17, 1668 CrossRef CAS PubMed.
- S. Lee, J. Hong, J. H. Koo, H. Lee, S. Lee, T.-j. Choi, H. Jung, B. Koo, H. Kim and J. Park, ACS Appl. Mater. Interfaces, 2013, 5, 2432 CAS.
- K. L. Zhang, Appl. Surf. Sci., 2012, 258, 7327 CrossRef CAS.
- Y. T. Xu, Y. Guo, L. X. Song, K. Zhang, M. M. F. Yuen, X. Z. Fu, R. Sun and C. P. Wong, RSC Adv., 2014, 4, 58005 RSC.
- M. Nasrollahzadeh, F. Babaei, P. Fakhric and B. Jaleh, RSC Adv., 2015, 5, 10782 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- J. Zhang, H. Yang, G. Shen, P. Cheng and S. Guo, Chem. Commun., 2010, 46, 1112 RSC.
- M. J. McAllister, J. L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, M. Herrera-Alonso, D. L. Milius, R. Car, R. K. Prud'homme and I. A. Aksay, Chem. Mater., 2007, 19, 4396 CrossRef CAS.
- Y. Sun, L. Xu, Z. l. Yin and X. Y. Song, J. Mater. Chem. A, 2013, 1, 12361 CAS.
- Y. Zhao, X. Song, Q. Song and Z. Yin, CrystEngComm, 2012, 14, 6710 RSC.
- W. Qu, H. Bao, L. Zhang and G. Chen, Chem–Eur. J., 2012, 18, 15746 CrossRef CAS PubMed.
- Y. Ping, J. Yan, Z. Wang, H. Wang and Q. Jiang, J. Mater. Chem. A, 2013, 1, 12188 CAS.
- E. de Smit, F. M. de Groot, R. Blume, M. Hävecker, A. K-Gericke and B. M. Weckhuysen, Phys. Chem. Chem. Phys., 2010, 12, 667–680 RSC.
- M. Zhang, D. Lei, X. Yu, L. Chen, Q. Li, Y. Wang, T. Wang and G. Cao, J. Mater. Chem., 2012, 22, 23091 RSC.
- Y. Qin, X. Ji, J. Jing, H. Liu, H. Wu and W. Yang, Colloids Surf. A, 2010, 372, 172 CrossRef CAS.
- D. V. Goia, J. Mater. Chem., 2004, 14, 451 RSC.
- X. Li, W. Qi, D. Mei, M. L. Sushko, I. Aksay and J. Liu, Adv. Mater., 2012, 24, 5136 CrossRef CAS PubMed.
- M. Willander, K. Ul Hasan, O. Nur, A. Zainelabdin, S. Zaman and G. Amin, J. Mater. Chem., 2012, 22, 2337 RSC.
- B. Nam, H. J. Lee, H. Goh, Y. B. Lee and W. San Choi, J. Mater. Chem., 2012, 22, 3148 RSC.
- W. Chen and T. Zhang, Nanoscale, 2013, 5, 1564 RSC.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- B. Adhikari, A. Biswas and A. Banerjee, ACS Appl. Mater. Interfaces, 2012, 4, 5472 CAS.
- H. Liu and Q. Yang, J. Mater. Chem., 2011, 21, 11961 RSC.
- G. T. Fu, X. Jiang, L. F. Ding, L. Tao, Y. Chen, Y. W. Tang, Y. M. Zhou, S. H. Wei, J. Lin and T. H. Lu, Appl. Catal., B, 2013, 138, 167 CrossRef.
- G. Fu, L. Ding, Y. Chen, J. Lin, Y. Tang and T. Lu, CrystEngComm, 2014, 16, 1606 RSC.
- R. Zhao, M. X. Gong, H. M. Zhu, Y. Chen, Y. W. Tang and T. H. Lu, Nanoscale, 2014, 6, 9273 RSC.
- J. Li, C. Y. Liu and Y. Liu, J. Mater. Chem., 2012, 22, 8426 RSC.
- H. T. Fu, X. H. Yang, X. C. Jiang and A. B. Yu, Langmuir, 2013, 29, 7134 CrossRef CAS PubMed.
- Y. Tian, Y. Y. Cao, F. Pang, G. Q. Chen and X. Zhang, RSC Adv., 2014, 4, 43204 RSC.
- J. Huang, S. Vongehr, S. Tang, H. Lu, J. Shen and X. Meng, Langmuir, 2009, 25, 11890 CrossRef CAS PubMed.
- L. Y. Chen, J. S. Yu, T. Fujita and M. W. Chen, Adv. Funct. Mater., 2009, 19, 1221 CrossRef CAS.
- S. Murphy, L. B. Huang and V. Kamat Prashant, J. Phys. Chem. C, 2013, 117, 4740 CAS.
- O. Mabayoje, M. Seredych and T. J. Bandosz, ACS Appl. Mater. Interfaces, 2012, 4, 3316 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05186c |
| This journal is © The Royal Society of Chemistry 2016 |