Mesoporous nanocrystalline TiO2 loaded with ferulic acid for sunscreen and photo-protection: safety and efficacy assessment
Abstract
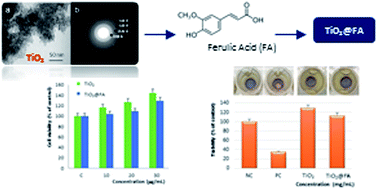
In the present study, the use of surfactant-free mesoporous TiO2 combined with an antioxidant and photo-protecting agent, such as ferulic acid (FA), as a sunscreen was investigated for the first time. Ferulic acid is a natural antioxidant characterized by UV absorption capacity and radical scavenging activities and, due to these properties, it has been approved as an active ingredient in several skin lotions and sunscreens. However, despite the double function exerted by FA, the use of this molecule in the cosmetic field is limited by its poor stability. Aiming to overcome this drawback, mesoporous TiO2, prepared by using a sol–gel route assisted by a polyoxyethylene–polyoxypropylene block copolymer template followed by solvothermal treatment, was used as a matrix for the encapsulation of ferulic acid. The stability studies performed confirmed the ability of the prepared material to preserve the active molecule from degradation induced by light and, therefore, its properties. Antioxidant and anti-inflammatory activities of FA-loaded titania (TiO2@FA) and titania matrix (TiO2) were evaluated and high scavenging activity towards DPPH, ABTS and NO radicals were recorded. The in vitro assessment of the spectrophotometric Sun Protection Factor (SPF) was also performed and a value of 14.7 was observed for TiO2@FA while mesoporous TiO2 showed a lower SPF value equal to 2.6. These results suggested the potential application of the titania-doped FA as a “booster of SPF” that is able to enhance the SPF of a sunscreen. Furthermore, in vitro safety studies confirmed the biocompatibility of the prepared material and the absence of skin irritation.

 Please wait while we load your content...
Please wait while we load your content...