Preparation of microcapsules containing multi-functional reactive isocyanate-terminated-polyurethane-prepolymer as healing agent, part II: corrosion performance and mechanical properties of a self healing coating
M. Haghayegh,
S. M. Mirabedini* and
H. Yeganeh
Iran Polymer and Petrochemical Institute, P.O. Box: 14965/115, Tehran, Iran. E-mail: sm.mirabedini@ippi.ac.ir; Fax: +98 21 4866 2054; Tel: +98 21 4866 2401
First published on 17th May 2016
Abstract
The preparation of microcapsules made of a polyurethane (PU) shell, and multi-functional isophorone diisocyanate based polyurethane prepolymer core as healing agent (BIH) was reported in the first part of this work. The preparation of epoxy coatings loaded with PU-based microcapsules, the self-healing ability of these coatings, the resistance of self-healed coatings against corrosive media and the mechanical strength of these healed coatings are described in the present communication. For comparison, a similar coating system, loaded with microcapsules containing monomeric isophorone diisocyanate (IPDI) core as healing agent, was also prepared and subjected to the same evaluations. For reliable comparison of these two coating systems and to show that the nature of the healing agent was the sole determining factor on the recorded results, the same reactive core fraction, solvent content, shell material and shell wall thickness were considered for the prepared microcapsules. In addition, an equal weight percent of the added microcapsules was used for both systems. The salt spray test and electrochemical impedance spectroscopy (EIS) techniques were utilized to show the ability of these two systems to heal a cracked area artificially created in the coatings. The recorded results confirmed the superior ability of multi-functional high molecular weight isophorone diisocyanate based healing agents for the protection of damaged areas of coating against corrosive media. The ability of a healing agent to repair the coating material was also verified by comparing the tensile strength of the coatings before and after crack formation and the healing of the cracked area, respectively. The epoxy coating could regain up to 95% of its original tensile strength, when loaded with 10 wt% of microcapsules containing multi-functional isophorone diisocyanate based polyurethane prepolymer as healing agent.
1. Introduction
Polymeric coatings are well-known for their function in protecting underlying metallic substrates and this may be achieved if the coatings remain intact at service time. However, organic polymeric coatings are usually unavoidably subjected to unforeseen cracks and/or damage through their service life.1 Mechanical and/or physical defects can be also created during the process of producing the coatings. Therefore, improvement in the coatings' ability to withstand undesirable environmental factors such as corrosive media and mechanical stresses is a prerequisite for development of durable high performance coatings.2,3The barrier property of the coatings and their ability to protect underlying substrates from external mechanical stresses can be enhanced in different ways such as; increasing the thickness and cross link density of the coatings,4–6 applying multi layer coatings7 and adding nanoparticles/nanoplates.1–3,8–10 However, the performance of the best available coatings can also deteriorate when they are damaged by external forces. Therefore, preserving/returning to the original performance of the coatings is a crucial factor for extending the usability of these protective layers. To fulfil this need, many coatings with the ability to heal their damaged areas automatically and autonomously were developed by scientists.11,12 There are different ways to produce self-healing coatings, but incorporation into the coatings' formulations of microcapsules containing a healing agent is one of the most studied approaches. In this case, the active agent released from the ruptured microcapsules can repair the damage and continue the role of the protective layer.13–20
Recently, the use of reactive isocyanate based healing agents which can facilitate crack filling via a reaction with atmospheric moisture, under catalyst free conditions, has attracted attention. The encapsulation of isocyanates as either blocked or exposed forms into a PU shell through an oil-in-water emulsion method was reported.21,22 Encapsulation of hexamethylene diisocyanate (HDI) as a healing agent was also reported by Sottos et al.23 The corrosion performance of epoxy coatings containing this type of self-healing agent was studied later, utilizing salt spray and EIS techniques.24 The role of the shell chemical structure on the performance of this system was studied by Dicredico25 who prepared microencapsulated IPDI in either PU or polyurea–formaldehyde (PUF) single layer shells, as well as PU–PUF bilayer shells. As aforementioned, most of the reported studies utilized low molecular weight isocyanate molecules as healing agents.
Despite many advantages, the low rate of the curing reaction with atmospheric moisture under catalyst free conditions, and the low physical/chemical quality of the new coating produced over the damaged area are the main drawbacks of such systems. One approach for solving these problems is to use higher molecular weight multifunctional isocyanate molecules as healing agents. The optimized preparation and characterization of novel single-layer polyurethane type microcapsules, richly and efficiently loaded with bulky isocyanate molecules were described in the first part of this research.26 The microcapsules were loaded into an epoxy-based coating and the crack healing efficiency of the incorporated healing agent was studied and compared with a similar system loaded with microcapsules containing monomeric IPDI. The present study was designed to find out deeper information regarding the ability of the released healing agent to protect the exposed substrate against corrosive media and to regain the original tensile strength of the matrix coating material.
2. Experimental
2.1. Materials
Microcapsules with a PU shell, containing either BIH as the core material (MBIH), or IPDI as the core healing agent (MIPDI) were prepared according to the procedures reported in our previous publication.26 Toluene diisocyanate (TDI), isophorone diisocyanate (IPDI), 2-ethyl-2-hydroxy methyl-1,3-propandiol (TMP, trimethylolpropane), chlorobenzene (CB), 1,4-butandiol (BD), dibutyltin dilaurate catalyst (DBTL) and gum arabic (GA) were purchased from Merck. The methods followed for the chemical characterization of microcapsules, evaluation of their size and morphology, determination of the encapsulated solvent content as well as reactive isocyanate healing agents were fully described previously.26Epoxy resin, KER 828, based on diglycidyl ether of bisphenol A (DGEBA) and its amine containing hardener (CRAYAMID 140C) were received from HEXION Specialty Chemicals and Cray Valley, respectively. Cardura Hexion E-10P and EFKA 2722 were supplied from Kalakar Company, used as a plasticizer and an antifoaming agent, respectively, for different coating formulations.
2.2. Preparation of microcapsules
Two classes of microcapsules were prepared with the same polyurethane shell composed of TMP, IPDI and BD, containing either IPDI or BIH as core healing agents. The procedures followed for the preparation of these materials were fully described in the first part of this work.262.3. Preparation of epoxy coatings containing MBIH or MIPDI as healing agents
Different amounts of microcapsules (1, 3, 5, 10, 15 and 20 wt%), 0.5 wt% EFKA 2722 and 3 wt% Cardura Hexion E-10P were gently added into the KER 828, under magnetic stirring for 10 min. The samples were placed in a low pressure oven for 10 min to remove trapped air. A stoichiometric amount of the hardener, CRAYAMID 140C, with a resin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hardener weight ratio of 2
hardener weight ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, was added to the coating formulation, and additionally stirred for 5 min. The samples were then applied on either degreased mild steel substrates or PTFE sheets using a film applicator (Zehntner ZUA2000 universal applicator 2390–3000 μm) with a wet film thickness of 500 ± 20 μm and final dry thickness of 325 ± 25 μm (measured using Mega check 20-ST, Fe–NFe). After exposure to the air for 2 h and post curing for 30 min at 80 °C, the coating samples applied on the PTFE sheets were gently removed from the substrate. The free-standing films were then cut in dimensions of 2 × 10 cm2 and the strips were left for about 48 h at ambient temperature, post cured for 2 h at 80 °C and used for tensile strength measurements. Coating samples on mild steel substrates were left for 30 min at ambient temperature, for 30 min at 80 °C and finally for 48 h at ambient temperature, and used for natural salt spray test and EIS studies.
1, was added to the coating formulation, and additionally stirred for 5 min. The samples were then applied on either degreased mild steel substrates or PTFE sheets using a film applicator (Zehntner ZUA2000 universal applicator 2390–3000 μm) with a wet film thickness of 500 ± 20 μm and final dry thickness of 325 ± 25 μm (measured using Mega check 20-ST, Fe–NFe). After exposure to the air for 2 h and post curing for 30 min at 80 °C, the coating samples applied on the PTFE sheets were gently removed from the substrate. The free-standing films were then cut in dimensions of 2 × 10 cm2 and the strips were left for about 48 h at ambient temperature, post cured for 2 h at 80 °C and used for tensile strength measurements. Coating samples on mild steel substrates were left for 30 min at ambient temperature, for 30 min at 80 °C and finally for 48 h at ambient temperature, and used for natural salt spray test and EIS studies.
2.4. Tensile strength properties of the filled coatings
The tensile strengths of the coating samples containing microcapsules (filled with either multi-functional IPDI or IPDI monomers) were studied using a Santam STM-20 Universal testing machine, according to ASTM D 882 standard test practice. The crosshead moved with a load cell of 500 N and a constant speed of 5 mm min−1 at 23 ± 2 °C. For each formulation, at least three replicate samples were tested for statistical accuracy. A crack was created on some of the free-standing strip films using a scalpel blade, of 2 cm length, parallel to the direction of tension and about 100 μm depth. The tensile strength test was performed before, immediately and 24 h after crack creation. The healing efficiency of the microcapsule embedded coating samples was calculated by comparing the tensile properties of the samples prior to and after crack creation.2.5. Corrosion performance studies
To verify the effect of microcapsules on the corrosion performance of epoxy-based coated mild steel specimens, natural salt spray test chamber and EIS techniques were conducted. Natural salt spray chamber test: two cross scratch lines of 5 cm length were created on the coated sample using a scalpel blade according to the procedure described in ASTM D 1653. The scratched specimen was then left for 24 h at ambient temperature for the healing process. Before corrosion performance tests, the back and edges of the samples were covered with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt% mixture of molten colophony resin and beeswax. Both neat coated samples and the samples containing different amounts of either multi-functional IPDI or IPDI monomer filled microcapsules (1, 3, 5, 10, 15 and 20 wt%) were examined in this study. The specimens were exposed to a 50 g L−1 NaCl solution at 35 ± 1 °C according to the ASTM B 117 test procedure for 30 days. Samples were visually examined and photographed periodically during the test.
1 wt% mixture of molten colophony resin and beeswax. Both neat coated samples and the samples containing different amounts of either multi-functional IPDI or IPDI monomer filled microcapsules (1, 3, 5, 10, 15 and 20 wt%) were examined in this study. The specimens were exposed to a 50 g L−1 NaCl solution at 35 ± 1 °C according to the ASTM B 117 test procedure for 30 days. Samples were visually examined and photographed periodically during the test.
3. Results and discussion
Investigation of published research results regarding application of encapsulated isocyanate compounds (mainly low molecular weight analogous) as healing agents reveals that the rate of crack healing is not satisfactory for this system. This is the way in most of these studies; evaluation of the self-healing process was performed when these coatings were immersed into an aqueous solution instead of exposing them to atmospheric moisture, i.e. the real condition for the practical application of such systems. Meanwhile, due to the brittle nature of the polyurea formed upon the curing reaction of low molecular weight isocyanate based healing agents, the ability of the new coatings formed at the crack area is not satisfactory for the proper protection of the underlying substrate.27 This phenomenon is related to low molecular weight IPDI monomers with higher NCO content (38%) in comparison to higher molecular weight BIH molecules with lower NCO content (16%). Actually, the formation of networks with higher crosslinked density and shorter crosslinked chain length is expected for a system with an IPDI healing agent. Therefore, the application of a high molecular weight NCO-terminated polyurethane prepolymer composed of TMP and IPDI was considered in this work. In the first part of this study, the preparation and encapsulation of this compound into a polyurethane shell were reported. The efficient and accelerated crack filling ability of this type of healing agent in comparison to low molecular weight monomeric IPDI under atmospheric moisture conditions was described accordingly.26 In addition, preliminary evaluation of corrosion protection of the healed coatings containing either MBIH or MIPDI filled microcapsules revealed the superior performance of the former system. In the present work, the corrosion performance and mechanical properties of the healed coatings with two types of microcapsules are described.3.1. Preparation and characterization of synthesized microcapsules
The TDI and IPDI pre-polymers were synthesized with NCO contents of 15.7 and 21.3%, respectively.26 PU-based microcapsules filled with either bulky multi-functional IPDI or IPDI monomers, were synthesized via interfacial polymerization of TDI pre-polymer with BD as chain extender in an oil-in-water emulsion. The different conditions of the microcapsules' preparation were comprehensively studied in our previous work.26 To compare the healing ability of microcapsules with different core materials in the polymeric-based matrix, it is essential to keep constant all variables such as; the amount and viscosity of the available healing agent for filling the crack region. This can be possible, if the strength of the microcapsules' shell which is directly related to the shell thickness,28 the amount of the encapsulated reactive healing agent and the content of imprisoned solvent in the microcapsules, which determines its viscosity and as a result, its ability to flow through the crack were similar in the two types of microcapsule. The effects of variation in mixing speed and surfactant concentration on the reactive core fraction wt%, solvent content and shell thickness for two types of microcapsule in six different preparations with core/shell ratios of around 90/10 are presented in Table 1. Based on the results, microcapsules with IPDI pre-polymer as core material synthesized with an agitation rate of 750 rpm and surfactant concentration of 22 wt%, and the PU microcapsules with IPDI monomers as core material synthesized with an agitation rate of 600 rpm and surfactant concentration of 11 wt% were selected for comparison of their healing abilities. In these conditions, the amount of reactive core material, solvent content and shell thickness are around 43 wt%, 13.5 wt% and 1.9 μm, respectively for both types of microcapsules. In the case of microcapsules filled with IPDI monomers, with increasing concentration of surfactant, the microcapsules were not formed. Huang and Yang29 showed that the mean diameter and shell thickness of the PU microcapsules filled with HDI decreased with increasing surfactant concentration before reaching a critical micelle concentration (CMC) and after that became constant. Here, it seems that with increasing surfactant concentration, pre-formed microcapsules with very thin shell thickness were ruptured as a result of stress caused by mixing during the microcapsule preparation.| Mixing speed (rpm)/surfactant (wt%) | Core content (wt%) | Solvent content (wt%) | Shell thickness (μm) | |||
|---|---|---|---|---|---|---|
| Multi-functional IPDI | IPDI monomer | Multi-functional IPDI | IPDI monomer | Multi-functional IPDI | IPDI monomer | |
| 500/11 | 48.7 | 50 | 8.8 | 13.5 | 4.10 | 2.2 |
| 600/11 | 46.5 | 43.5 | 11.1 | 13.3 | 3.59 | 1.9 |
| 750/11 | 45.2 | 39.1 | 10.6 | 18.2 | 3.48 | 1.38 |
| 850/11 | 43.9 | 33.9 | 13.1 | 24.4 | 2.53 | 0.71 |
| 750/22 | 43.3 | — | 13.5 | — | 1.88 | — |
| 750/33 | 47.2 | — | 8.9 | — | 4.09 | — |
The optical and SEM micrographs of microcapsules containing two types of core materials are shown in Fig. 1. Both types are poly-dispersed spherical particles with sizes ranging from about 5 to 200 μm without any inter-capsule bonding. As shown in Fig. 1a, the microcapsules are perfectly spherical with a size range of 10–100 μm, average diameter of 45 μm and shell thickness of about 1.88 μm. As shown in the optical microscope image, the microcapsules containing multi-functional IPDI compounds are opaque compared with their counterparts with IPDI monomer core materials (Fig. 1b) which are transparent, probably due to the higher molecular weight of the core materials in comparison to IPDI monomers.
 | ||
| Fig. 1 Optical microscopes and SEM micrographs of PU-based microcapsules containing; (a) multi-functional IPDI, and (b) IPDI monomers. | ||
The microcapsules are individually distributed without excessive inter-capsule bonding. The outer and inner surfaces of the microcapsules' shells are smooth as is seen in other reports about polyurethane microcapsules.25,30 The surface and shell morphology of microcapsules containing IPDI monomers are shown in Fig. 1b. The microcapsules are almost spherical with a size range of 5–200 μm, average diameter of 60 μm and shell thickness of about 1.9 μm. As shown in Fig. 1a, the inner surface of the microcapsules is relatively smooth; however, the outer surface is wrinkled due to free space because of the lower molecular weight of the core material. As shown in the SEM micrographs, after application of low vacuum pressure during sample preparation for the SEM study, the surface of the microcapsules was deformed, causing the loss of their spherical shape, indicating that the PU shell is soft and deformable.25
In order to study the morphology of the core materials in two types of microcapsule, the cured epoxy coating containing the microcapsules was broken under liquid nitrogen and its cross section was evaluated using SEM. Fig. 2a and b show the cross section of the coating sample containing multi-functional IPDI filled microcapsules upon breaking and after 24 h exposure to ambient conditions (24.5 °C and 18.6% moisture). The SEM micrograph clearly shows curing of the core materials after 24 h aging in ambient conditions, with similar morphology and higher density compared with its counterpart upon rupturing (Fig. 2a). Fig. 2c shows the status of a ruptured microcapsule after 24 h exposure to ambient conditions. The micrograph clearly reveals completion of the curing of the core materials. Similar results were reported by Huang and Yang29 based on hexamethylene diisocyanate monomers as core materials, which are more active than IPDI monomers. However, to accelerate the curing process, the samples were immersed for 48 h in an aqueous solution of 10 wt% NaCl. To clarify the superiority of the healing ability of the multi-functional IPDI core in comparison to the monomeric one, the morphologies of the IPDI monomer core materials are shown in Fig. 2e–h. The SEM micrographs clearly showed that IPDI monomers couldn't cure at ambient conditions even after 48 h exposure to the air (Fig. 2e). The morphologies of the IPDI monomers after 6 and 10 days of exposure to the air are similar to those of microcapsules containing multi-functional IPDI just after breaking and after 24 h, respectively, Fig. 2f–h. Therefore, this shows that the curing process of reactive isocyanate monomers occurs over a long period of time just by exposure to ambient conditions.26 To solve this problem and to accelerate the healing process, the accelerated conditions (NaCl solution) were employed in the previous work. But it is desirable for the core material to have healing ability in ordinary conditions. Therefore, to omit the necessity of accelerated conditions for healing, an attempt has been made to design self-healing coatings based on microcapsules filled with bulky multi-functional isocyanates with higher molecular weight. The logic was based on the fact that the number of higher molecular weight units which are needed to join to each other to heal the crack is less in comparison to monomer units.26 Therefore, the rate of healing increases by using higher molecular weight material as the core material.26 In addition, in our previous work, films on the glass surface of 5 μm thickness were prepared from BIH and IPDI slides. These coated samples were placed under the same atmospheric conditions and the drying rates of these films were monitored. While tacking and curing times of about 15 min and 10 h were recorded for BIH, the IPDI film remained in liquid state even after 3 days.26 Based on the results presented in this section and in our previous work, it can be concluded that this strategy is efficient for creating effective healing properties. It is worth mentioning that increasing the molecular weight of the healing agent to much higher values is not suitable since it can also lead to increased viscosity of prepolymer and consequent decreased flowability of the healing agent into the damaged area of the coating. In the meantime, penetration of moisture into the healing agent may be influenced when a higher molecular weight healing agent is applied. These situations are not favored for the intended application of this class of isocyanate based materials as healing agent.
3.2. Mechanical properties of microcapsules-filled coating samples
Stress–strain curves of the coating samples containing various wt% of microcapsules containing multi-functional IPDI compounds are shown in Fig. 3a, and derived mechanical properties of the coatings are summarized in Table 2. In general, by increasing the microcapsule wt% from 1 to 10 wt%, the mechanical properties of the samples decreased (Fig. 3a). Investigation of the recorded tensile strength showed that the microcapsule loading had a negative effect on the overall tensile strength of the coatings. This phenomenon may be attributed to the microcapsules' aggregation and the consequent poor dispersion of this additive, as well as increasing the viscosity and trapping higher numbers of air bubbles in the epoxy resin matrix. Therefore, an increase in defects within the coating film caused a decrease in the overall mechanical properties, as also reported in the literature.29–31 Self-healing coatings with high healing performance are always desirable, while maintaining the mechanical properties.33 To achieve this goal, a balance between microcapsule loading and mechanical properties should be established.| Sample codingb | Elongation at break (%) | Ultimate strength (MPa) | Elastic modulus (MPa) | Work at break (MPa) |
|---|---|---|---|---|
| a The different superscript indices for each group of data (a, b, c, d and e) show that the p-value <0.05 and there are significant differences between the tensile data values in samples with different microcapsule loadings (confidence interval 0.95).b NE stands for neat epoxy sample, digit shows wt% of microcapsules in coating sample and B represents microcapsules with multi-functional IPDI compounds. | ||||
| NE | 13.44 ± 0.2a | 55.40 ± 3.4a | 1385 ± 30.19a | 505.02 ± 42.9a |
| 1BME | 11.75 ± 0.7b | 46.69 ± 1.9b | 1071 ± 56.26b | 327.33 ± 38.1b |
| 3BME | 8.94 ± 0.5c | 46.08 ± 1.7b | 991 ± 183.03b | 237.21 ± 20.9c |
| 5BME | 7.07 ± 0.3d | 42.16 ± 2.6b | 1252.75 ± 149.0a,b | 175.03 ± 18.4d |
| 10BME | 6.26 ± 0.2e | 34.52 ± 1.7c | 1127 ± 110.7b | 121.36 ± 10.7e |
Tensile strength test results were used to evaluate the self-healing efficiency of the coating samples. For this purpose, the tensile strength test was accomplished in three modes: intact, scratched and healed strip film samples. Fig. 3b–f show stress–strain curves for coating samples containing various wt% (0, 1, 3, 5 and 10) microcapsules with multi-functional IPDI core materials, in three different modes. In Table 3, mechanical properties of the microcapsule-filled coating samples in three different modes are tabulated.
| Sample coding | Sample mode | Elongation at break (%) | Ultimate strength (MPa) | Elastic modulus (MPa) | Work at break (MPa) |
|---|---|---|---|---|---|
| a The different superscript indices for each group of data (a, b, c, d and e) show that the p-value <0.05 and there are significant differences between the value of neat, scratched and healed samples (confidence interval 0.95). | |||||
| NE | Intact | 13.44 ± 0.2a | 55.40 ± 3.4a | 1385 ± 30.2a | 505.02 ± 43.0a |
| Scratched | 5.94 ± 0.3b | 37.85 ± 2.6b | 1299 ± 23.1b | 126.17 ± 16.4b | |
| Healed | 5.89 ± 0.5b | 38.02 ± 1.9b | 1300 ± 28.0b | 124.30 ± 15.5b | |
| 1BME | Intact | 11.75 ± 0.7a | 46.69 ± 1.9a | 1071 ± 56.3a | 327.33 ± 38.1a |
| Scratched | 5.34 ± 0.2b | 29.01 ± 2.6b | 1221 ± 74.1b | 88.99 ± 17.3b | |
| Healed | 7.82 ± 0.7c | 34.64 ± 4.2b | 1232 ± 22.1b | 146.73 ± 23.7c | |
| 3BME | Intact | 8.94 ± 0.5a | 46.08 ± 1.7a | 991 ± 183.0a | 237.21 ± 20.9a |
| Scratched | 5.09 ± 0.3b | 40.08 ± 0.6b | 1377.75 ± 162.9b | 112.79 ± 10.9b | |
| Healed | 7.20 ± 0.2c | 40.15 ± 1.1b | 1221 ± 54.1a,b | 160.85 ± 14.3c | |
| 5BME | Intact | 7.07 ± 0.3a | 42.16 ± 2.6a | 1252.75 ± 149.0a | 175.03 ± 18.4a |
| Scratched | 3.56 ± 0.6b | 29.03 ± 3.6b | 1363.25 ± 77.9a | 53.43 ± 9.6b | |
| Healed | 6.34 ± 0.1c | 36.18 ± 4.5a,b | 1287.25 ± 103.2a | 127.65 ± 10.4c | |
| 10BME | Intact | 6.26 ± 0.2a | 34.52 ± 1.7a | 1127 ± 110.7a | 121.36 ± 10.7a |
| Scratched | 4.53 ± 0.26b | 30.87 ± 0.73b | 1346.25 ± 130.4a | 77.28 ± 9.9b | |
| Healed | 5.99 ± 0.42c | 33.55 ± 1.83a | 1310.25 ± 163.4a | 109.91 ± 11.2a | |
The reported tensile data in Tables 2 and 3 are the mean value ± standard deviation of at least 3 samplings estimated by spss-17 software. One-way ANOVA has been employed by considering the post hoc Tukey option. The confidence interval value is 0.95.
3.3. Healing efficiency of microcapsules embedded coating samples
In microcapsule-based self healing systems, most research is into the effect of microcapsules on mechanical properties.29–31 Based on previous work, there are different methods to achieve healing efficiency. One of the most common methods is to use a fracture toughness test.33,34 Guadagno et al.35 reported a healing efficiency of 97.75% for a sample containing 10 wt% microcapsules by using a fracture toughness test. Es-haghi et al.36 used a water vapor transmission test (WVT) to calculate healing efficiency. Pingkarawat et al.37 used tension and compression tests for calculating healing efficiency in mendable composites containing self-healing thermoplastic agents. The results show that tension mode is better than compression mode to achieve healing efficiency. Guadagno et al.35 employed tensile strength results for this purpose. Therefore, tensile tests of intact, scratched and healed strip film samples were used to calculate healing efficiency for samples containing different amounts of microcapsules. In this study, the healing efficiency of the coating samples was calculated using both elongation at break and fracture toughness (eqn (1) and (2)):
 | (1) |
 | (2) |
The healing efficiencies for coating samples containing various wt% microcapsules with multi-functional IPDI core materials are shown in Fig. 4. The results revealed that the healing efficiency increased with an increase in the microcapsule loading from about 39 to 83% for coating samples containing 1 and 10 wt% BME microcapsules, respectively. This result was predictable because the amounts of healing agent increased due to the increasing microcapsule loading. Therefore, the healing efficiency increased. As a result of this, the crack filled with higher amounts of healing agent which was extracted from more ruptured microcapsules. Fig. 4b shows the healing efficiencies obtained from the fracture toughness for coating samples containing various wt% of microcapsules. A similar trend is observed from the fracture toughness compared with the results gained from the elongation at break. Healing efficiency increased from 24 to 74% for coating samples containing 1 and 10 wt% microcapsules. The results obtained from fracture toughness are more precise, because it is a better measure of the mechanical properties of the coating film. The ability of a coating film to absorb energy during elongation up to breaking point is the most important mechanical property of the sample.
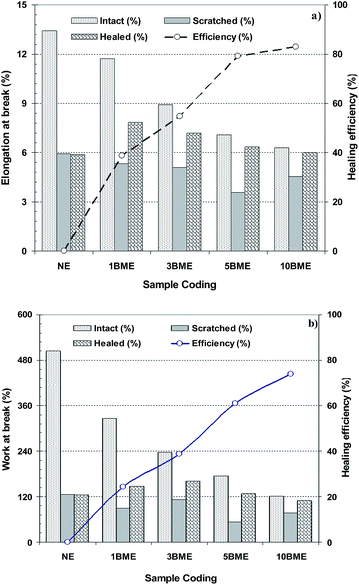 | ||
| Fig. 4 Healing efficiency for coating samples containing various wt% microcapsules with multi-functional IPDI core materials, based on comparison of; (a) elongation at beak, (b) work at break. | ||
SEM images of the healed region in the epoxy films containing different amounts of multi-functional IPDI filled microcapsules are shown in Fig. 5. These SEM images show that the crack was filled more in the film containing more microcapsules, which confirmed the tensile results.
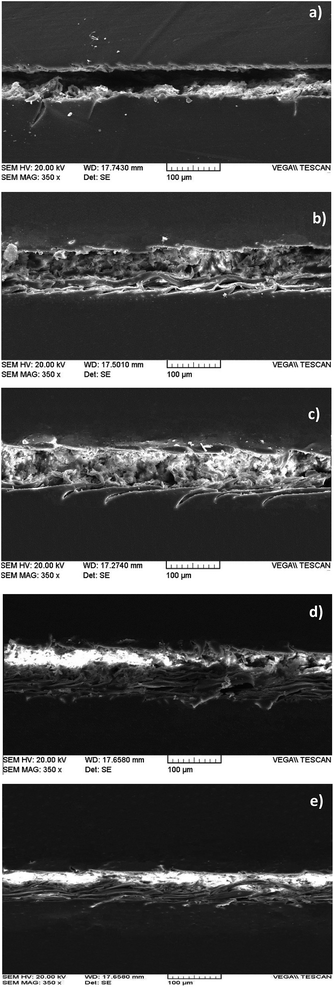 | ||
| Fig. 5 SEM micrographs of scratched area of epoxy films with various microcapsule loadings: (a) neat epoxy film, (b–e) films with 1, 3, 5 and 10 wt% microcapsule loading, respectively. | ||
As shown in Fig. 5e, the volume of the healed region is greater than the crack volume in the film with 10 wt% microcapsule loading. This swelling is probably the result of trapping higher amounts of CO2 which was produced by the reaction of a higher amount of released healing agent out of the ruptured microcapsules. The synthetic route for polyurea formation at the damaged area of coating includes partial conversion of isocyanate groups to corresponding amine groups and the reaction of the generated amines with remaining isocyanate functions. CO2 gas is a byproduct which is produced during the aforementioned reactions.25 This gas tends to escape to the air during polyurea formation. Since the formation of the polyurea layer and CO2 gas formation occur simultaneously, therefore, trapping of released gas within the polymeric layer can occur. For epoxy films containing higher amounts of microcapsules, there are higher amounts of released isocyanate compound and, therefore, the foaming process is more obvious in this system.
An attempt was made to apply a tensile test for samples containing microcapsules filled with an IPDI monomer core. The tensile data in non-scratched mode for different samples are similar to those for samples containing multi-functional IPDI filled microcapsules and the data in scratched and healed modes didn't show significant differences even after 48 h of the healing period. This result was related to the lower rate of curing reaction for IPDI monomers in comparison to BIH core, as previously mentioned.26
3.4. Corrosion performance of coating samples
The salt spray test method is widely used for corrosion performance evaluation;24 however, this test method can be also used to study the self healing properties of the coatings.24,30 When a microcapsule-filled coating film is exposed to mechanical stresses, cracks or damage, the microcapsules are ruptured, resulting in the release of the core materials. The released material fills the crack area and reacts with the surrounding coating, and hence restores the mechanical properties of the host coating. Release of core content may also enhance barrier properties in the cracked area of the polymeric film, by minimizing the water and oxygen permeability through the coating layer,3,18 resulting in delay to or retardation of the corrosion reactions.32 In this part of study, to make clear the differences between the healing abilities of two types of core materials (IPDI monomers and IPDI pre-polymer), the salt spray method was employed. Fig. 6 shows visual observations of the scratched area of coating samples containing different wt% of microcapsules containing multi-functional IPDI or IPDI monomer core materials, during 30 days in the salt spray test chamber. The results revealed that application of either type of microcapsule improves the corrosion resistance of the coating samples and retards the rusting of the scratched region. The level of corrosion hindrance depends on the loading and type of core material of the microcapsules in the coating formulation. For the neat epoxy sample, rusting has been observed in the scratched area and developed with progressive exposure time. Fig. 6a shows the coatings containing bulky multi-functional IPDI-filled microcapsules. The corrosion process began after 2 days for the sample containing 1 and 3 wt% microcapsules, and after 14, 21 and 30 days for the samples having 5, 10 and 15 wt% microcapsules, respectively. No sign of corrosion or rusting was observed for the sample containing 20 wt% microcapsules, even after 30 days of exposure to the salt spray test conditions. Fig. 6b shows the coating with IPDI monomer-filled microcapsules. The rusting of the exposed scratched area had begun after one day for samples containing 1 and 3 wt% microcapsules and after 2 days for samples containing; 5, 10, 15 and 20 wt% microcapsules. Hence, these results indicate two facts: the first one is the improvement in the corrosion protection with an increase in the microcapsule loading. Higher microcapsule loading leads to higher amounts of released healing materials. As a result of this, a thicker protective layer can be formed in the crack region.24,32 Huang and Yang24 reported that the corrosion resistance of epoxy coatings containing HDI-filled microcapsules is enhanced by increasing microcapsule loading and coating thickness. The second fact is that the bulky multi-functional IPDI material is more effective than its counterpart with an IPDI monomer core content in retarding the corrosion process of the scratched epoxy coating sample. As mentioned earlier, the reaction of the higher molecular weight units is faster than that of the monomer ones. Therefore, the cured bulky IPDI pre-polymers have higher molecular weight, higher crosslinked density, resulting in delay in water and oxygen penetration through the healed region and enhanced corrosion resistance of the layer. | ||
| Fig. 6 Visual observation of scratched coating samples containing different wt% of: (a) multi-functional IPDI-filled microcapsule and (b) IPDI monomers, in different salt spray exposure times. | ||
The EIS method is widely used for corrosion studies32,38,39 and it can be also used for evaluating the self healing properties of the coatings.40–43 The EIS technique is more efficient than the natural salt spray test, because it can evaluate the corrosion process before the appearance of any sign of rusting or blistering on the coating film. The presence of the charge carrier ions affects the resistance of the coating. Therefore, the value of coating resistivity can be used to evaluate the corrosion protection of the coating and EIS is a practical technique for this function.44
The Nyquist and Bode plots for the scratched areas of the neat coating samples and samples containing 5 wt% microcapsules filled with multi-functional IPDI or IPDI monomer core materials during 30 days immersion in 3.5 wt% NaCl electrolyte are shown in Fig. 7 and 8, respectively. Based on the tensile measurement and salt spray test results, coating samples containing 5 wt% microcapsules were selected as the optimum conditions for the EIS study. The results show that with increasing immersion time, the diameter of the semi-circle in Nyquist plots (Fig. 7), dramatically decreased as a result of diffusion of the charge carrier ions through the scratched area and reduced resistivity of the samples. The resistivity reduction is more evident for the neat epoxy sample due to the absence of any protective layer to hinder the movement of ions. The sample containing microcapsules with multi-functional IPDI core material revealed a lower decrease in the coating resistance during the exposure time.
Randle's model45 consisting of three components – electrolyte resistance, Rs, resistance component, Rp, and constant phase element (Q) – was considered to be an appropriate equivalent circuit for simulating the Nyquist plots. The results of fitting a Nyquist plot with Randle's model are tabulated in Table 4. Higher corrosion resistances for microcapsule embedded coating samples, Rp = 213.5 and 207 kΩ for IPDI monomer and bulky multi-functional IPDI, respectively, compared with neat epoxy coating (Rp = 29 kΩ), were observed. The core materials released out of ruptured microcapsules relatively healed the crack to form a protective layer between electrolyte solution and steel substrate. The Rp value decreased from 207 to 104.6 kΩ for the samples containing bulky multi-functional IPDI, from 213.5 to 29.3 kΩ for coating containing IPDI monomer and from 29 to 6.46 kΩ for neat epoxy coatings after 2 weeks immersion in NaCl electrolyte, due to penetration of the electrolyte through the healed layer. It is clear that the bulky IPDI healing agent can heal the crack better than the IPDI monomer and forms a protective layer with a higher resistance in comparison to IPDI monomers.
| Immersion time (h) | Coating resistance (kohm) | ||
|---|---|---|---|
| Neat epoxy sample | Coating with 5 wt% MIPDI loading | Coating with 5 wt% MBIH loading | |
| 0.25 | 29 | 213.5 | 207 |
| 1 | 28.86 | 221.8 | 198.8 |
| 4 | 28.6 | 164.6 | 160.7 |
| 24 | 19.93 | 68.1 | 151.8 |
| 48 | 11.44 | 63.9 | 148.2 |
| 168 | 7.19 | 39.3 | 122.6 |
| 336 | 6.46 | 29.3 | 104.6 |
| 720 | 2.44 | 10.81 | 19.59 |
The Rp for all coating samples were also obtained from Bode plots at low frequency, averaged over at least three measurements, in the plateau region.39 The variation in Rp for all samples during 30 days immersion time is tabulated in Fig. 9. The corrosion resistance of the healed region decreased with increasing time, owing to penetration of water and ions within the formed layer coating, which depended on the nature of the healing agent.
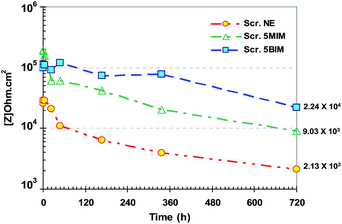 | ||
| Fig. 9 Resistive components, Rp, calculated from Bode plots for all scratched samples during 720 h immersion in 3.5 wt% NaCl electrolyte. | ||
The resistivity of the scratched neat epoxy sample is relatively higher than that of the bare metal, Z = 2.13 kΩ cm2. This can be attributed to possible changes happening in the scratched region, such as swelling of the coating's cut edges and build up of corrosion products in the scratched area, which cause ion transfer through the barrier-formed complex and in doing so improves the resistivity of the compound. For samples containing multi-functional IPDI or IPDI monomers, the Rp was obtained as Z = 24.3 and 9.03 kΩ cm2, respectively. IPDI monomers show somewhat low corrosion resistance in comparison to multi-functional IPDI due to lower Mw and a weaker formed film.
4. Conclusion
PU-based microcapsules filled with bulky multi-functional IPDI and IPDI monomers as core materials were successfully synthesized via an interfacial polymerization method. In both cases, the IPDI fraction, core content and shell thickness were measured as 43 wt%, 13.5 wt% and 1.9 μm, respectively. Optical and scanning electron microscopy micrographs revealed that the microcapsules filled with IPDI monomers are optically transparent with wrinkled outer and smooth inner surfaces, while the bulky multi-functional IPDI-filled microcapsules were relatively opaque due to the presence of higher Mw core materials. The results of several test methods revealed that the bulky multi-functional IPDI can heal the damage better and faster than IPDI monomers due to the higher Mw. The tensile measurement results showed that 95% healing efficiency was achieved by 10 wt% microcapsules filled with bulky IPDI core materials. Salt spray and EIS methods also confirmed the better and faster healing ability of the bulky multi-functional IPDI in comparison to IPDI monomers.References
- X. M. Tong, T. Zhang, M. Z. Yang and Q. Zhang, Colloids Surf., A, 2010, 371, 91–97 CrossRef CAS.
- C. Berry, B. J. Hicks, C. J. McPherson, A. J. Medland and G. Mullineux, Packag. Technol. Sci., 2005, 18, 225–241 CrossRef.
- S. M. Mirabedini, I. Dutil and R. R. Farnood, Colloids Surf., A, 2012, 394, 74–84 CrossRef CAS.
- I. Hackman and L. Hollaway, Composites, Part A, 2006, 37, 1161–1170 CrossRef.
- B. C. Kim, S. W. Park and D. G. Lee, Compos. Struct., 2008, 86, 69–77 CrossRef.
- M. Alexandre and P. Dubois, Mater. Sci. Eng., 2000, 28, 1–63 Search PubMed.
- G. G. Miller, J. L. Kepler and D. Darwin, ACI Struct. J., 2003, 100, 314–320 CrossRef.
- A. Yasmin, J. L. Abot and I. M. Daniel, Scr. Mater., 2003, 49, 81–86 CrossRef CAS.
- A. Yasmin, J. J. Luo, J. L. Abot and I. M. Daniel, Compos. Sci. Technol., 2006, 66, 2415–2422 CrossRef CAS.
- R. Sarathi, R. K. Sahu and P. Rajeshkumar, Mater. Sci. Eng., A, 2007, 445–446, 567–578 CrossRef.
- M. A. Shenoy and D. J. D'Melo, Surf. Coat. Int., Part B, 2006, 89, 221–230 CrossRef CAS.
- G. Pan, Q. Guo, Z. Zhao, S. Wang, Y. Qin and L. Wang, Tribol. Int., 2011, 44, 789–796 CrossRef CAS.
- R. F. A. Teixeira, X. K. D. Hillewaere, S. Billiet and F. E. Du Prez, Macromol. Rapid Commun., 2013, 34, 290–309 CrossRef PubMed.
- S. K. Ghosh, Self-Healing Mater., 2009, 1–291 Search PubMed.
- X. Feng, G. Zhang, B. Xu, H. Jiang, Q. Bai and H. Li, RSC Adv., 2015, 5, 70000–70004 RSC.
- J. S. Monfared Zanjani, B. S. Okan, I. Letofsky-Papst, Y. Menceloglu and M. Yildiz, RSC Adv., 2015, 5, 73133–73145 RSC.
- E. N. Brown, S. R. White and N. R. Sottos, J. Mater. Sci., 2004, 39, 1703–1710 CrossRef CAS.
- E. Brown, N. R. Sottos and S. R. White, Exp. Mech., 2002, 42, 372–379 CrossRef CAS.
- J. D. Rule, E. N. Brown, N. R. Sottos, S. R. White and J. S. Moore, Adv. Mater., 2005, 17, 205–208 CrossRef CAS.
- D. Sondari, A. A. Septevani, A. Randy and E. Triwulandari, Int. J. Eng. Technol., 2010, 2, 466–471 CAS.
- M. W. Keller, S. R. White and N. R. Sottos, Adv. Funct. Mater., 2007, 17, 2399–2404 CrossRef CAS.
- M. M. Caruso, D. A. Delafuente, V. Ho, N. R. Sottos, J. S. Moore and S. R. White, Macromolecules, 2007, 40, 8830–8832 CrossRef CAS.
- M. R. Kessler, N. R. Sottos and S. R. White, Composites, Part A, 2003, 34, 743–753 CrossRef.
- M. Huang and J. Yang, Prog. Org. Coat., 2014, 77, 168–175 CrossRef CAS.
- B. Di Credico, M. Levi and S. Turri, Eur. Polym. J., 2013, 49, 2467–2476 CrossRef CAS.
- M. Haghayegh, S. M. Mirabedini and H. Yeganeh, J. Mater. Sci., 2016, 51, 3056–3068 CrossRef CAS.
- K. Holzworth, Z. Jia, A. V. Amirkhizi, J. Qiao and S. Nemat-nasser, Polymer, 2013, 54, 3079–3085 CrossRef CAS.
- Y. Jinglei, M. W. Keller, J. S. Moore, S. R. White and N. R. Sottos, Macromolecules, 2008, 41, 9650–9655 CrossRef.
- M. Huang and J. Yang, J. Mater. Chem., 2011, 21, 11123 RSC.
- X. Liu and J. K. Lee, Adv. Mater., 2011, 239–242, 1794–1798 CrossRef CAS.
- A. Matsuzaki, M. Nagoshi, H. Noro, M. Yamashita and N. Hara, Mater. Trans., 2011, 52, 1244–1251 CrossRef CAS.
- A. Stankiewicz, I. Szczygie and B. Szczygie, J. Mater. Sci., 2013, 48, 8041–8051 CrossRef CAS.
- M. Behzadnasab, S. M. Mirabedini, K. Kabiri and S. Jamali, Corros. Sci., 2011, 53, 89–98 CrossRef CAS.
- J. D. Rule, N. R. Sottos and S. R. White, Polymer, 2007, 48, 3520–3529 CrossRef CAS.
- L. Guadagno, M. Raimondo, C. Naddeo, P. Longo, A. Mariconda and W. H. Binder, Smart Mater. Struct., 2014, 23, 045001 CrossRef.
- H. Es-Haghi, S. M. Mirabedini, M. Imani and R. R. Farnood, Composites, Part B, 2016, 85, 305–314 CrossRef CAS.
- K. Pingkarawat, C. H. Wang, R. J. Varley and A. P. Mouritz, Composites, Part A, 2014, 65, 10–18 CrossRef CAS.
- A. Czeller and T. Czigány, ECCM15 – 15th European Conference on Composite Materials, Venice, Italy, 24–28 June 2012 Search PubMed.
- M. Behzadnasab, S. M. Mirabedini and M. Esfandeh, Corros. Sci., 2013, 75, 134–141 CrossRef CAS.
- R. Gharibi, M. Yousefi and H. Yeganeh, Prog. Org. Coat., 2013, 76, 1454–1464 CrossRef CAS.
- E. M. Fayyad, M. A. Almaadeed, A. Jones and A. M. Abdullah, Int. J. Electrochem. Sci., 2014, 9, 4989–5011 Search PubMed.
- W. Wang, L. Xu, X. Li, Z. Lin, Y. Yang and E. An, J. Mater. Chem. A, 2014, 2, 1914 CAS.
- H. Zhang, J. Wang, X. Liu, Z. Wang and S. Wang, Ind. Eng. Chem. Res., 2013, 52, 10172–10180 CrossRef CAS.
- D. G. Shchukin, M. Zheludkevich, K. Yasakau, S. Lamaka, M. G. S. Ferreira and H. Möhwald, Adv. Mater., 2006, 18, 1672–1678 CrossRef CAS.
- A. Lasia, Electrochemical Impedance Spectroscopy and Its Applications, Modern Aspects of Electrochemistry, ed. B. E. Conway, J. Bockris and R. E. White, Kluwer Academic/Plenum Publishers, New York, 1999, vol. 32, pp. 143–248 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |




