Open Access Article
Open Access Article“Clickable” thiacalix[4]arene derivatives bearing polymerizable 1,3-butadiyne fragments: synthesis and incorporation into polydiacetylene vesicles†
Vladimir
Burilov
*a,
Alsu
Valiyakhmetova
a,
Diana
Mironova
a,
Roman
Safiullin
c,
Marsil
Kadirov
bc,
Kamil
Ivshin
a,
Olga
Kataeva
ab,
Svetlana
Solovieva
ab and
Igor
Antipin
ab
aKazan Federal University, 18 Kremlevskaya st, Kazan, 420008, Russian Federation. E-mail: ultrav@bk.ru; Fax: +7-843-238-79-01; Tel: +7-843-2337344
bA.E.Arbuzov Institute of Organic & Physical Chemistry, 8 Arbuzov str., Kazan, 420088, Russian Federation
cKazan National Research Technological University, 68 Karl Marx str., Kazan, 420015, Russian Federation
First published on 3rd May 2016
Abstract
p-tert-Butylthiacalix[4]arene derivatives in 1,3-alternate stereoisomeric form bearing polymerisable 1,3-butadiyne fragments on the one side and amino/carboxylic groups on another were synthesized using stepwise functionalisation. Calixarene 7 embedded in polydiacetylene nanoparticles showed a selective colorimetric response toward lanthanide ions.
Recently, polymeric materials that undergo spectral changes in response to external mechanical stimuli have gained much attention.1,2 A conjugated polymer, polydiacetylene (PDA), has a special place among such materials. PDA can be prepared from various kinds of self-assembled diacetylene monomers by simple photo polymerization3 and can be used for colorimetric detection of ions,4 chemicals,5 biomolecules,6,7 bacteria8,9 and pH10 or temperature changes.11 The key mechanism of the PDA colorimetric response lies in distortion of the π-conjugated backbone by external stimuli and appears as rapid color (blue-to-red) and fluorescence changes (none-to-red).12 Functional groups on the surface of PDA nanoparticles or bilayer play the key role in colorimetric detection of target substrates.
(Thia)calix[4]arene derivatives are well-known objects of supramolecular chemistry with unique properties: variety of stereoisomeric configurations, easy functionalization of both upper and lower rim and preorganization effect.13,14 Their ability to form host–guest complexes and to bind both organic molecules and metal ions is successfully used in extraction; recognition of different substrates and as components of molecular devices.15–17 Azide- or terminal alkyne functionalities can essentially extend the synthetic potential of calixarene platform by using of the copper-catalyzed azide-alkyne cycloaddition (CuAAC).18–20
So, the combination of unique receptor properties of calixarene derivatives with PDA as a signaling unit is very challenging in creation of colorimetric sensors. Thus, charged calixarene receptors, non-covalently embedded within vesicles comprising phospholipids and the PDA were successfully used for the color fingerprinting of proteins.21 Nevertheless direct covalent bounding of calixarene with PDA signaling unit is at high interest but represented only in covalent incorporation of calixarenes with PDA matrix via ester-linkage,22 which is not hydrolytically stable in acidic/base conditions or in the presence of carboxylic ester hydrolases.23 It is noteworthy that thiacalix[4]arenes scaffold adopting 1,3-alternate stereoisomeric form is in a great importance since selective stepwise functionalization of macrocycle lower rim allows to create two molecular domains with quite different properties located on the opposite sides from macrocycle plane (Scheme 1). One of them provides polymerisable 1,3-butadiynic fragments; on other the target receptor fragments can be easily introduced and varied using click chemistry approach.
Herein we report a new synthetic strategy for the wide series of thiacalix[4]arene – based bifunctional receptors in 1,3-alternate stereoisomeric form bearing polymerizable 1,3-butadiyne fragments on the one side and azidopropyl fragments for the CuAAC reaction on another and the study of colorimetric response toward d-metal ions by receptors embedded in 10,12-pentacosadiynoic acid (PCDA) vesicles.
Mitsunobu reaction is the most convenient method for the stepwise functionalization of thiacalix[4]arene lower rim by two different electrophilic reagents and leads to non-symmetrical tetra-substituted products in 1,3-alternate stereoisomeric form24,25 containing different functional groups on the opposite sides of the macrocycle (Scheme 2).
The stereoselective synthesis of polymerisable diazido precursor 3 was accomplished in a convergent fashion. The synthesis utilizes the 5-phenyl-2,4-pentadiyn-1-ol and 3-azido-1-propanol as building blocks. Building blocks were synthesized by Cadiot–Chodkiewicz coupling reaction of propargyl alcohol with phenyl acetylene in the presence of Cu(I) chloride26 and nucleophilic substitution of 3-bromo-1-propanol with sodium azide, correspondingly.
Obviously, there are two pathways (A or B on the Scheme 2) to the target precursor, that differ in the sequence of reactions. On the first step the substituents bearing terminal azide or 1,3-butadiynoic fragments were introduced on the macrocycle. Reactions of parent thiacalix[4]arene with 5-phenyl-2,4-pentadiyn-1-ol and 3-azido-1-propanol lead to the formation of expected distal disubstituted derivatives 1 and 2 in good yields. It is important to note that no Staudinger by-products (reaction of azides with triphenyl phosphine) were detected. So, azido-containing fragments can be directly attached to calixarene platform in Mitsunobu reaction conditions. Earlier azide-groups were only introduced on the thiacalix[4]arene platform by nucleophilic substitution of corresponding halogen derivatives.25
In this way thiacalixarene 3 was obtained by Mitsunobu reaction of 1 or 2 with 3-azido-1-propanol and 5-phenyl-2,4-pentadiyn-1-ol, respectively, and there was no significant difference in the yields. So, the sequence of the reactions has no effect on the total yield of the final product.
Structures of compounds 1–3 were characterized by NMR, MALDI-TOF, IR and elemental analysis (see ESI†). For compound 1 single crystals suitable for X-ray diffraction studies were obtained. According to the X-ray data O-substituted aromatic rings of the macrocycle are parallel to each other (Fig. 1). We found that it is general observation for distal disubstituted thiacalix[4]arene derivatives.27,28 Moreover, this is completely different from the behavior of disubstituted classical calix[4]arene (C[4]A) derivatives,29 in which both O–R fragments are fixed by intramolecular hydrogen bonding resulting in the rigid cup-shaped structure.
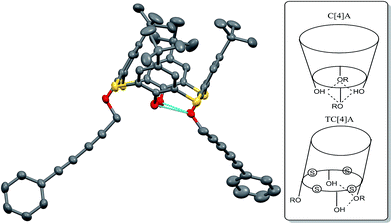 | ||
| Fig. 1 Structure of 1 according to the X-ray data (hydrogen atoms are omitted for clarity) and intramolecular hydrogen bonding in C[4]A and TC[4]A derivatives. | ||
The reason of such behavior in disubstituted TC[4]A derivatives is 15% larger ring size of thiacalix[4]arene,30 that's why only one phenolic hydroxyl group can take part in hydrogen bonding (Fig. 1).
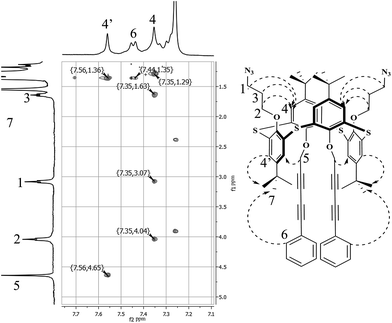
Stereoisomeric form of 3 was assigned as 1,3-altenate by the presence of high symmetric structure confirmed by 1H NMR spectra and 2D NOESY NMR due to the existence of cross-peaks between signals of methylene protons of azidopropyl fragments (δ = 4.04; 3.07; 1.63 ppm) and neighboring aromatic protons (δ = 7.35 ppm) as well as cross-peaks between signals of methylene protons of 5-phenylpenta-2,4-diyn fragments (δ = 4.65 ppm) and neighboring aromatic protons (δ = 7.56 ppm) (Fig. 2).
Reaction of calixarene 3 with several alkynes was performed both in Cu(I)- catalyzed (for compounds 4, 5) and uncatalyzed (for 7) conditions (Scheme 3). Reaction of 3 with acetylenedicarboxylic acid gave tetraacid 7, reaction with N-propargyl phthalimide following by hydrazinolysis gave diamine 6 with good yields. No products of calixarene 3 self-cyclization were found. Low reactivity of disubstituted 1,3-butadiyne derivatives in the cycloaddition reaction with azides31 is a reason of such result.
 | ||
| Scheme 3 Synthesis of triazoles 4–7, reaction conditions: CuI, NEt3, toluene, RT (for 4, 5); acetone, reflux (7) and NH2–NH2·H2O, EtOH, reflux (6). | ||
Calixarenes 6 and 7 were used in copolymerization with 10,12-pentacosadiynoic acid (PCDA), which is the most often used PDA source capable for vesicle formation.32 Despite low topochemical matching of diynoic fragments of calixarene and PCDA it is quite expected that calixarenes can form functional domains on the vesicle surface after polymerization. PCDA–calixarene vesicles were formed by well-known film hydration method. According to DLS data (Table 1) PCDA itself forms 200 nm sized vesicles, addition of anionic calixarene 7 at pH 7.4 doesn't significantly affect to the size of vesicles, but addition of calixarene 6, which is mostly in cationic form at pH 7.4, causes high aggregation of PCDA. Polymerization of PCDA–calixarene vesicles was performed under irradiation of 254 nm light in quarts 10 mm cuvettes was controlled by UV-visible spectroscopy. The maximum absorbance peak at 674 nm, which corresponds to the “blue” form of PDA vesicles, appeared after 15 minutes of irradiation, and then polymer began to convert in “red” form. Thus 15 minutes is the optimal time for complete polymerization of PDA vesicles. The size of vesicles after polymerization decreases in all cases at about 30% (Table 1). Zeta-potential of polymerized vesicles is in full accordance with the nature of calixarene dopants. In the case of anionic calixarene 7ξ-potential of negatively charged PDA vesicles is increased up to −62 mV, whereas the presence of cationic calixarene 6 leads to its decreasing (−37 mV).
According to the TEM data (Fig. 3) the shape of polymerized PDA vesicles has both spherical and tapered forms with size 150–200 nm, PDA doped with calixarene 6 has 200 nm single tapered particles and shapeless aggregates, and PDA doped with calixarene 7 has spherical shape particles with 100 nm in diameter. Non-spherical shape can be the main reason of high polydispersity indexes in DLS measurements.
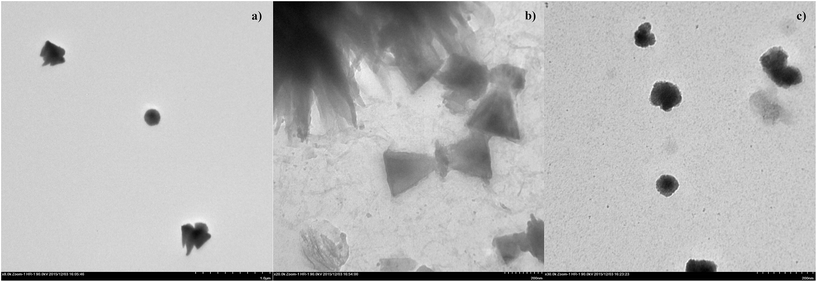 | ||
| Fig. 3 TEM images of polymerized PDA vesicles (a), PDA-6 (b) and PDA-7 (c) vesicles, C(PCDA) = 0.2 mM, C(calix) = 0.02 mM. | ||
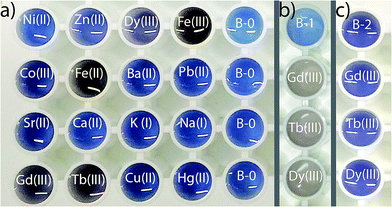
Since PDA nanomaterial's with different functional groups on the surface were successfully used in detection of such ions as lead, silver, zinc, cadmium, manganese, silver, mercury,33–35 the study of the effect of calixarene doping on the colorimetric response of PDA vesicles in the presence of different metal ions was done. According to the obtained data (Fig. 4a) PDA-7 vesicles with 10% mol content of calixarene has a colorimetric response to lanthanide ions (Gd3+, Tb3+ and Dy3+ were tested). Importantly that addition of other metal ions (except iron(II) and (III) ions that's colored due to the partial hydrolysis at pH 7.4) has no color effect on PDA-7 vesicles solution. Therefore selectivity upon lanthanide ions can be successfully used for lanthanide determination in the presence of other metal ions. The increase of calixarene content in PDA vesicles up to 20 mol% (Fig. 4b) lead to significant increase of the colorimetric response toward lanthanide ions. Minimum detection limit is 0.01 mM (see pic. 2 in ESI†).
It is noteworthy that PDA vesicles without calixarene additive (Fig. 4c) has no changes in absorption spectra upon addition of lanthanide ions. Moreover, the overall picture of colorimetric response for “pure” PDA vesicles is quiet differ – at the same concentrations they have response toward Sr(II), Ca(II) and Pb(II) (pic. 1, ESI†). Thus, calixarene additive significantly changes affinity and selectivity of modified vesicles to metal ions. Recently36 we have found that calixarene with the same functional dicarboxytriazolyl fragments can bind the Tb(III) ion due to the great affinity of carboxylate groups to lanthanide ions.
It is well known that calixarenes are allosteric systems:37 conformational changes of the molecule upon binding of analytes infavors or disfavors the subsequent binding (respectively, in the case of positive or negative allosteric effect). This effect is achieved through the flexibility of calixarene cavity at both cone and 1,3-alternate stereoisomeric forms. It often used for calixarene-based sensors containing receptor and indicator functions on the opposite sides of macrocycle plane.38,39 So, the selective colorimetric response upon addition of lanthanide ions can be attributed with the deformation of calixarene backbone caused by the strong complexation on the one side of calixarene platform and corresponding distortion of PDA π-conjugated backbone on other side of macrocycle.
Thus, suggested synthetic strategy for the thiacalix[4]arene – based bifunctional receptors can be a good background for the design of the wide series of colorimetric sensors with an easily tunable selectivity.
Conclusions
For the first time a new synthetic strategy for the synthesis of wide series of thiacalix[4]arene – based bifunctional receptors in 1,3-alternate stereoisomeric form bearing polymerizable 1,3-butadiyne fragments on the one side and azidopropyl fragments for the CuAAC reaction on another was demonstrated. Thiacalixarenes containing amino- and carboxylic groups were used in copolymerization with 10,12-pentacosadiynoic acid to give functional PDA nanoparticles. It was found that polymerized PDA vesicles with 20% mol content of calixarene 7 has significant colorimetric response toward lanthanide ions with the detection limit up to 0.01 mM. Great selectivity upon lanthanide ions can be a good basis for the analytical determination in the presence of other ions.Acknowledgements
TEM images were carried out in the laboratory of “Transmission electron microscopy” of Kazan National Research Technological University. We thank the Russian Scientific Foundation for the financial support of this work (grant No. 14-13-01151).Notes and references
- M. M. Ali, D. K. Kang, K. Tsang, M. Fu, J. M. Karp and W. Zhao, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2012, 4, 547–561 CrossRef CAS PubMed.
- H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79–93 RSC.
- S. Okada, S. Peng, W. Spevak and D. Charych, Acc. Chem. Res., 1998, 31, 229–239 CrossRef CAS.
- S. Kolusheva, T. Shahal and R. Jelinek, J. Am. Chem. Soc., 2000, 122, 776–780 CrossRef CAS.
- J. Jaworski, K. Yokoyama, C. Zueger, W. J. Chung, S. W. Lee and A. Majumdar, Langmuir, 2011, 27, 3180–3187 CrossRef CAS PubMed.
- S. Kolusheva, L. Boyer and R. Jelinek, Mol. Reprod. Dev., 2000, 264, 225–227 Search PubMed.
- C. H. Park, J. P. Kim, S. W. Lee, N. L. Jeon, P. J. Yoo and S. J. Sim, Adv. Funct. Mater., 2009, 19, 3703–3710 CrossRef CAS.
- Y. Scindia, L. Silbert, R. Volinsky, S. Kolusheva and R. Jelinek, Langmuir, 2007, 23, 4682–4687 CrossRef CAS PubMed.
- Z. Ma, J. Li, M. Liu, J. Cao, Z. Zou, J. Tu and L. Jiang, J. Am. Chem. Soc., 1998, 120, 12678–12679 CrossRef CAS.
- S. M. Ryu, I. Yoo, S. Song, B. Yoon and J. M. Kim, J. Am. Chem. Soc., 2009, 131, 3800–3801 CrossRef CAS PubMed.
- S. J. Kew and E. A. H. Hall, Anal. Chem., 2006, 78, 2231–2238 CrossRef CAS PubMed.
- M. A. Reppy and B. A. Pindzola, Chem. Commun., 2007, 4317–4338 RSC.
- Z. Asfari, V. Bohmer, J. Harrowfield and J. Vicens, Calixarenes 2001, Kluver, Netherland, 2001 Search PubMed.
- R. Kumar, Y. O. Lee, V. Bhalla, M. Kumar and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4824–4870 RSC.
- M. Giuliani, I. Morbioli, F. Sansone and A. Casnati, Chem. Commun., 2015, 51, 14140–14159 RSC.
- N. Morohashi, F. Narumi, N. Iki, T. Hattori and S. Miyano, Chem. Rev., 2006, 106, 5291–5316 CrossRef CAS PubMed.
- S. E. Solovieva, R. A. Safiullin, E. N. Kochetkov, N. B. Melnikova, M. K. Kadirov, E. V. Popova, I. S. Antipin and A. I. Konovalov, Langmuir, 2014, 30, 15153–15161 CrossRef CAS PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS PubMed.
- N. V. Sokolova and V. G. Nenajdenko, RSC Adv., 2013, 3, 16212–16242 RSC.
- V. A. Burilov, R. I. Nugmanov, E. V. Popova, I. R. Nabiullin, S. E. Solovieva, I. S. Antipin and A. I. Konovalov, Macroheterocycles, 2014, 7, 10–17 CrossRef.
- S. Kolusheva, R. Zadmard, T. Schrader and R. Jelinek, J. Am. Chem. Soc., 2006, 128, 13592–13598 CrossRef CAS PubMed.
- D. L. Dermody, Y. Lee, T. Kim and R. M. Crooks, Langmuir, 1999, 15, 8435–8440 CrossRef CAS.
- F. Hasan, A. A. Shah and A. Hameed, Enzyme Microb. Technol., 2006, 39, 235–251 CrossRef CAS.
- I. Bitter and V. Csokai, Tetrahedron Lett., 2003, 44, 2261–2265 CrossRef CAS.
- V. A. Burilov, R. I. Nugmanov, R. R. Ibragimova, S. E. Solovieva and I. S. Antipin, Mendeleev Commun., 2015, 25, 177–179 CrossRef CAS.
- W. Chodkiewicz, Ann. Chim., 1957, 2, 819–869 CAS.
- S. K. Latypov, S. V. Kharlamov, A. A. Muravev, A. A. Balandina, S. E. Solovieva, I. S. Antipin and A. I. Konovalov, J. Phys. Org. Chem., 2013, 26, 407–414 CrossRef CAS.
- S. E. Solovieva, E. V. Popova, A. O. Omran, A. T. Gubaidullin, S. V. Kharlamov, Sh. K. Latypov, I. S. Antipin and A. I. Konovalov, Russ. Chem. Bull., 2011, 60, 486–498 CrossRef CAS.
- L. C. Groenen, E. Steinwender, B. T. G. Lutz, J. H. van der Maas and D. N. Reinhoudt, J. Chem. Soc., Perkin Trans. 2, 1992, 1893–1898 RSC.
- H. Akdas, L. Bringel, E. Graf, M. W. Hosseini, G. Mislin, J. Pansanel, A. D. Cian and J. Fischer, Tetrahedron Lett., 1998, 39, 2311–2314 CrossRef CAS.
- I. N. Domnin, L. A. Remizova, G. L. Starova and F. Rominger, Russ. J. Org. Chem., 2009, 45, 1678–1682 CrossRef CAS.
- S. Okada, S. Peng, W. Spevak and D. Charych, Acc. Chem. Res., 1998, 31, 229–239 CrossRef CAS.
- J. Guo, L. Yang, L. Zhu and D. Chen, Polymer, 2013, 54, 743–749 CrossRef CAS.
- D. E. Wang, Y. Wang, C. Tian, L. Zhang, X. Han, Q. Tu, M. Yuan, S. Chen and J. Wang, J. Mater. Chem. A, 2015, 3, 21690–21698 CAS.
- D. A. Jose and B. König, Org. Biomol. Chem., 2010, 8, 655–662 CAS.
- V. A. Burilov, D. A. Mironova, R. R. Ibragimova, S. E. Solovieva, B. König and I. S. Antipin, RSC Adv., 2015, 5, 101177–101185 RSC.
- L. Kovbasyuk and R. Krämer, Chem. Rev., 2004, 104, 3161–3187 CrossRef CAS PubMed.
- M. Kumar, A. Dhir and V. Bhalla, Tetrahedron, 2009, 65, 7510–7515 CrossRef CAS.
- M. Kumar, R. Kumar and V. Bhalla, Tetrahedron Lett., 2010, 51, 5559–5562 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1463902. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra07555j |
| This journal is © The Royal Society of Chemistry 2016 |