Designing hierarchical NiO/PAni-MWCNT core–shell nanocomposites for high performance super capacitor electrodes
Abstract
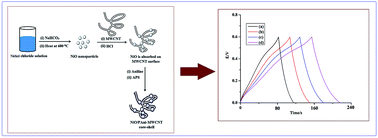
A novel type of nanocomposite material based on multi walled carbon nanotubes (MWCNT) and NiO nanoparticles coated with polyaniline (PAni) has been prepared by an in situ polymerization technique. Transmission electron microscopy confirms the core–shell structure of the composite. TG-analysis reveals that NiO/PAni-MWCNT nanocomposites exhibit better thermal stability than NiO-PAni. The synthesized nanocomposites have good conductivity and excellent electrochemical properties. The specific capacitance values of these composites were measured from their galvanostatic charge–discharge curves. The particles show a high value of specific capacitance and can find potential application as super capacitor electrodes.

 Please wait while we load your content...
Please wait while we load your content...