Hydrobromic acid assisted crystallization of MAPbI3−xClx for enhanced power conversion efficiency in perovskite solar cells†
Abstract
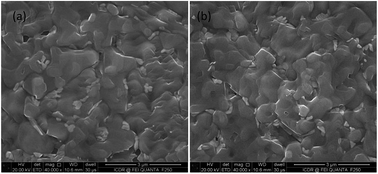
Enhanced performance of perovskite solar cells based on the application of high quality MAPbI3−xClx films developed via a hydrobromic acid assisted fast crystallization process is reported. A current density (Jsc) of 21.71 mA cm−2, an open circuit voltage (Voc) of 0.94 V, a fill factor (FF) of 0.77 and a high power conversion efficiency (PCE) of 15.76% were obtained. Noticeably, the hydrobromic acid assisted device exhibited less hysteresis and followed a crystallization route which is several times faster than that of the traditional one-step spin-coating method. The enhancement in device performance is attributed to the increased parallel resistance, lower leakage current, reduced series resistance and stronger crystallization of the MAPbI3−xClx perovskite layer.

 Please wait while we load your content...
Please wait while we load your content...