Mesoporous silica coated Gd2(CO3)3:Eu hollow nanospheres for simultaneous cell imaging and drug delivery†
Yanli Wua,
Xianzhu Xub,
Xi Chena,
Ruchun Yanga,
Qiang Xiao*a and
Yongxiu Li*c
aJiangxi Key Laboratory of Organic Chemistry, Jiangxi Science & Technology Normal University, Nanchang, 330013, China. E-mail: Xiao@tsinghua.org.cn; Fax: +86-791-83815183; Tel: +86-791-83815183
bCollege of Biology, Jiangxi Normal University, Nanchang 330022, China
cCollege of Chemistry, Nanchang University, Nanchang 330031, China. E-mail: yxli@nuc.edu.cn
First published on 22nd June 2016
Abstract
In the present work, mesoporous silica coated Gd2(CO3)3:Eu hollow nanospheres (Gd2(CO3)3:Eu@mSiO2 HNSs) were successfully synthesized via a facial route and characterized by X-ray diffraction (XRD), transmission electron microscope (TEM), scanning electron microscope (SEM) infrared spectrometer (IR), energy dispersive X-ray spectrum (EDS) and Brunauer–Emmet–Teller (BET) surface area analysis. The results indicate that the prepared monodispersed nanoparticles are hollow spheres with a 400 nm sphere core and 30 nm thick shell and have a narrow size distribution. In vitro cell imaging of the hollow nanosphere shows Gd2(CO3)3:Eu@mSiO2 HNSs were able to enter NCI-H460 lung cancer cells rapidly. The possibility of using the synthesized hollow nanospheres for magnetic resonance imaging was also demonstrated, and the hollow nanosphere displays a clear T1-weighted effect and could potentially serve as a bimodal T1-positive contrast agent. The drug loading and controlled release performance of Gd2(CO3)3:Eu@mSiO2 HNSs was evaluated with doxorubicin hydrochloride (DOX) as a model drug at different pH values (pH = 7.4, 5.8). The Gd2(CO3)3:Eu@mSiO2 HNSs showed sustainable pH dependent drug release property. Furthermore, the in vitro cytotoxic effect against NCI-H460 lung cancer cells of the DOX-loaded carriers was investigated in detail. In all, the Gd2(CO3)3:Eu@mSiO2 HNSs as a new type of theragnostic (imaging and treatment) agent can provide new opportunities in cancer treatment.
1 Introduction
The design and synthesis of multifunctional nanomedical platforms that integrate multiple suitable nanomaterials with different properties into one single nanosystem provides an unparalleled opportunity for the simultaneous diagnostics and therapy of diseases.1–3 For example, hollow multifunctional nanomaterials with low densities and high surface-to-volume ratios have been used for drug delivery to improve therapeutic efficiency and reduce toxicity.4–7 Hollow structures have gained particular attentions in the field of drug storage and release because of their large voids inside the shells and mesopores in the shells.8 The large voids can store more drug molecules than the conventional mesoporous materials, and the mesopores in the shells provide accessible channels for drug molecule diffusion and mass transfer without blocking. It is worth noting that the multimodal mesoporous nanoparticles suitable for optical, magnetic resonance (MRI) imaging, and drug delivery can be realized by the hollow and mesoporous drug delivery system by incorporating multiple imaging probes.9–13 The multimodal mesoporous nanoparticles are of great interest because they ally the highly sensitive fluorescence imaging and the high spatial resolution of MRI with controlled drug release.In recent years, much research attention has been paid to the rare earth ions doped Gd-based nanocrystals due to their unique magnetic and optical characteristics arising from their 4f electrons. Highly water-dispersible Gd-based nanoparticles, such as GdPO4,14–17 NaGdF4,18,19 and Gd2O3,20,21 have been reported as the doping matrices of luminescent ions (Eu3+, Tb3+, Yb3+/Er3+) and magnetic ions (Gd3+) that possess seven unpaired electrons and can efficiently alter the relaxation time of the surrounding water protons. However, their cytotoxicity has been rarely discussed. Recently, Fosrenol (lanthanum carbonate, La2(CO3)3) has been approved as a phosphate binder for the treatment of hyperphosphatemia in renal dialysis patients in both USA and Europe,22 indicating the promising biological application prospect of Gd2(CO3)3.23,24 In our previous work, the rare earth ions doped Gd2(CO3)3 nanoparticles were prepared by a reverse microemulsion method, and used as the dual modal agent for optical and magnetic resonance imaging.24 The chemical similarities of lanthanide elements indicate that the rare earth ions doped Gd2(CO3)3 nanoparticles might be safe for clinical applications. Therefore, it is expected that the hollow-structured Gd2(CO3)3 would provide more space for drug loading due to the hollow spherical core and nanopore channels and thus it is more attractive than the conventional Gd2(CO3)3 nanoparticles. And so far, there is no report on preparing hollow structured Gd2(CO3)3:Eu for biomedical application.
Many efforts have been made to develop efficient methods for the design and preparation of hollow nanostructures during the past decade. Template-directed synthesis method has been demonstrated to be an effective approach to prepare inorganic hollow spheres. Hard templates, such as polymer latex particles and carbon spheres and silica,25,26 and soft templates, such as emulsion droplets, micelles, and gas bubbles,27–29 are the two kinds of templates widely used in the template-directed synthesis method.
In the present work, we have developed a new strategy for the fabrication of mesoporous silica coated Gd2(CO3)3:Eu hollow nanospheres (Gd2(CO3)3:Eu@mSiO2 HNSs, Scheme 1). The hollow nanospheres were prepared by a homogeneous precipitation method using urea as a precipitating agent and silica nanosphere as a hard template, followed by a NaOH etching treatment. The hollow nanospheres were subsequently coated with a thin layer of silica and refluxed to remove the directing agent CTAB for the pore evolution and channel formation. The structure, formation process, luminescence and paramagnetic properties of the as-obtained nanostructure were investigated in detail. In addition, the high drug loading capacity and controlled drug release property of the prepared hollow-structured nanoparticles were demonstrated with doxorubicin hydrochloride (DOX) as a model drug.
2 Experimental
2.1 Materials
Gd2O3 (99.99%), Eu2O3 (99.99%), tetraethyl orthosilicate (TEOS) (99.0%), and RECl3 (RE = Gd and Eu) salts were freshly prepared by the reaction of RE2O3 with dilute hydrochloric acid. All other chemicals were of analytical-grade and used as received without further purification.2.2 Synthesis of silica cores
The highly monodispersed silica nanospheres (NSs) were synthesized by a modified Stöber method.30,31 The hydrolysis of tetraethoxysilane (TEOS) by ammonia in an ethanol/water solution yields the colloidal solution of silica nanoparticles with a narrow size distribution under optimal conditions. In a typical preparation, the mixture of 3 mL TEOS and 47 mL EtOH was added to a 50 mL ammonia solution in EtOH/H2O with a NH3·H2O (25 wt%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH volume ratio of 5
EtOH volume ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 and stirred at room temperature for 5 h to form a white silica colloidal suspension. The silica particles were collected by centrifugation and washed with ethanol four times. The particle size of SiO2 nanospheres were tuned by adjusting the amount of TEOS.
35 and stirred at room temperature for 5 h to form a white silica colloidal suspension. The silica particles were collected by centrifugation and washed with ethanol four times. The particle size of SiO2 nanospheres were tuned by adjusting the amount of TEOS.
2.3 Synthesis of core–shell SiO2@Gd2(CO3)3:Eu NPs
To prepare core–shell SiO2@Gd2(CO3)3:Eu nanoparticles, 9.5 mmol GdCl3 and 0.5 mmol EuCl3 were dissolved in 200 mL distilled water and then mixed with 3.0 g urea and 3.0 g PVP under vigorous stirring to form a clear solution. The as-prepared SiO2 nanospheres (400 mg) were well-dispersed in the above solution with the assistance of 10 min ultrasonication. The SiO2 nanosphere suspension was transferred into a 500 mL round-bottom flask and heated at 90 °C for 6 h under vigorous stirring. The produced core–shell product was collected by centrifugation and washed with distilled water and ethanol for three times, respectively, and dried at 60 °C.2.4 Synthesis of Gd2(CO3)3:Eu HNSs
The as-prepared core–shell structured SiO2@Gd2(CO3)3:Eu NPs (600 mg) was added to 20 mL 15% NaOH and stirred overnight to remove the SiO2 core. The hollow Gd2(CO3)3:Eu NSs were washed with distilled water several times for further use.2.5 Synthesis of Gd2(CO3)3:Eu@mSiO2 HNSs
The precursor of Gd2(CO3)3:Eu HNs was then coated with a silica layer by a modified Stöber process. The prepared Gd2(CO3)3:Eu HNSs (300 mg) were dispersed in 200 mL solution containing CTAB (300 mg) in EtOH–H2O solution (v/v, 160/40) under ultrasonication. One milliliter 25% ammonia and 300 μg TEOS were subsequently added to the Gd2(CO3)3:Eu HNSs suspension dropwise and stirred at room temperature for 12 h. The nanoparticles were collected by centrifugation, sequentially washed with deionized water and EtOH, and dried at 60 °C. The directing agent (CTAB) was extracted from the Gd2(CO3)3:Eu hollow nanospheres as described in the previous report.32 The dense silica coated precursor (200 mg) was dispersed in 50 mL acetone and refluxed at 75 °C for 10 h. The CTAB-removed product was collected by centrifugation, washed with acetone twice, and dried at 80 °C for 12 h. The product was denoted as Gd2(CO3)3:Eu@mSiO2.2.6 Characterization
The X-ray diffractions (XRD) of the powder samples were examined on a diffractometer (D8 Advance, Bruker, Germany). The morphologies of the products were imaged with a scanning electron microscopy (Quanta 200F, FEI, Hillsboro, USA) equipped with Energy dispersive X-ray spectrometer (GENESIS, EDAX, USA) and a transmission electron microscopy (Tecnai G20, FEI, Hillsboro, USA) equipped with a field emission gun operating at 200 kV. IR spectra were recorded on a Perkin-Elmer C99957 IR spectrophotometer (Perkin-Elmer, USA). The N2 adsorption/desorption isotherm was measured with a Micromeritics ASAP 2010M instrument at liquid nitrogen temperature (77 K). The specific surface area was determined by the Brunauer–Emmett–Teller (BET) method. Dynamic light scattering (DLS) measurements were carried out using a particle size analyzer (NPA152, Microtrac, USA). Photoluminescence was examined under an F4500 fluorescent spectroscopy (Hitachi, Japan) and magnetic measurements were carried out with a vibrating sample magnetometer in the range from −20 to 20 kOe (MPMS XL-7, Quantum Design Inc, USA). The absorption spectra were obtained with an UV/VIS spectrometer (Agilent 8453, USA).2.7 Cell imaging
NCI-H460 lung cancer cells were plated on 14 mm glass coverslips, allowed to adhere for 24 h, washed with phosphate buffer solution (PBS), and incubated in a serum-free cell culture medium containing 100 μg mL−1 Gd2(CO3)3:Eu@mSiO2 HNSs at 37 °C under 5% CO2 for 4 h. The excessive nanoparticles were removed by sufficiently washing the cells with PBS. Fluorescence images of the cells were collected on a confocal microscopy (ZEISS 710, Zeiss, Germany) under excitation at 405 nm.In vitro T1-weighted MRI and longitudinal relaxivity. The T1-weighted MR images were obtained using a 0.5 T magnet (Shanghai Niumag Corporation NM 120-Analyst). The r1 was calculated according to the equation r1 = ΔR1/[Gd3+ concentration, where R1 was the longitudinal relaxation rate (R1 = 1/T1, unit of s−1)]. In this study, R1 values of Gd3+ with different concentrations (0–1.0 mM−1).
2.8 Drug loading and drug release
Doxorubicin hydrochloride (DOX), a typical antitumor drug, was used as a model drug to evaluated the drug loading and controlled release performance of Gd2(CO3)3:Eu@mSiO2 HNSs. Briefly, 10 mg of as-prepared Gd2(CO3)3:Eu@mSiO2 HNSs was dispersed in 4 mL of PBS (pH = 7.4) with a DOX concentration of 0.5 mg mL−1. The DOX loaded Gd2(CO3)3:Eu@mSiO2 HNSs sample was collected by centrifugation. To evaluate the DOX loading amount, the supernatant solution was collected, and the content of residual DOX was determined by UV-vis measurement at a wavelength of 480 nm, as shown in Fig. S3 ESI.†The in vitro delivery test was performed by immersing the DOX-loaded Gd2(CO3)3:Eu@mSiO2 sample in 4 mL of PBS. At predetermined time intervals, PBS was quickly taken and replaced with an equal volume of fresh PBS. The amount of released DOX in the supernatant solution was measured by UV-vis spectrophotometer, the supernatant and washed solutions were collected and the residual DOX content (RDox) was obtained by UV-vis measurement at 480 nm. The loading efficiency of DOX can be calculated as follows: [(ODox − RDox)/ODox] × 100%, in which ODox is the original DOX content.
In vitro cytotoxicity against NCI-H460 lung cancer cells with free DOX and DOX-loaded Gd2(CO3)3:Eu@mSiO2.
Lung cancer cells were plated out in 96-well plates at a density of 8000 cells per well and were allowed to the well for 24 h. The free DOX, DOX-loaded Gd2(CO3)3:Eu@mSiO2 were added to the medium, the cells were incubated in 5% CO2 at 37 °C for 24 h. The concentrations of DOX were 0, 3.125, 6.25, 12.5, 25, and 50 μg mL−1, respectively. Finally, the cell viability was evaluated by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.
2.9 Biocompatibility of the Gd2(CO3)3:Eu@mSiO2 HNSs
The biocompatibility of the Gd2(CO3)3:Eu@mSiO2 HNSs was assessed by the standard MTT assay. The typical procedure and results were showed in ESI.†3 Results and discussion
3.1 Formation process, phase identification, and morphology
The morphology and structure of the samples were characterized by SEM, TEM, XRD, and EDS. Fig. 1 shows the SEM images of silica NSs, core-shell-structured SiO2@Gd2(CO3)3:Eu, and the Gd2(CO3)3:Eu HNSs. Fig. 2 shows the TEM images of core–shell-structured SiO2@Gd2(CO3)3:Eu, Gd2(CO3)3:Eu HNSs, and Gd2(CO3)3:Eu@mSiO2 HNSs, and the HRTEM of the Gd2(CO3)3:Eu@mSiO2 HNSs. It is clear that silica NSs are uniform and monodisperse nanoparticles with smooth surfaces and an average diameter of 400 nm (Fig. 1a). It is noted that the uniform core–shell-structured SiO2@Gd2(CO3)3:Eu nanospheres inherited the spherical morphology and good dispersibility of the silica templates. However, their surfaces are much rougher than that of the silica template due to core–shell-structure formed by the precipitation of a large number of uniform nanoparticles (Fig. 1b). Their panoramic SEM image indicates that Gd2(CO3)3:Eu HNSs are well-dispersed hollow nanospheres with diameters of ∼400 nm (Fig. 1c). These results reveal that the shape and structure of the final products essentially depend on the silica template. | ||
| Fig. 1 SEM images of pure SiO2 (a), core–shell-structured SiO2@Gd2(CO3)3:Eu (b), and Gd2(CO3)3:Eu HNSs (c). | ||
 | ||
| Fig. 2 TEM images of core–shell-structured SiO2@Gd2(CO3)3:Eu (a), Gd2(CO3)3:Eu HNSs (b), and Gd2(CO3)3:Eu@mSiO2 HNSs (c), and the HRTEM image of Gd2(CO3)3:Eu@mSiO2 HNSs (d). | ||
The TEM image of core–shell-structured SiO2@Gd2(CO3)3:Eu (Fig. 2a) further shows the rough surfaces and solid structure of the precursor nanospheres with a diameter of 400 nm, consistent with the SEM result (Fig. 1b). The TEM image of the Gd2(CO3)3:Eu HNSs (Fig. 2b) indicates their uniform spherical morphology. The strong contrast between the dark edge and the pale center is the direct evidence for the hollow structure of the nanospheres. The Fig. 2c show that the Gd2(CO3)3:Eu@mSiO2 HNSs inherit the spherical morphology with diameter of about 400 nm and good dispersity of the precursor which was also identified by the DLS (as shown in Fig. S1 ESI†). HRTEM images of the mesoporous silica coated Gd2(CO3)3:Eu clearly demonstrate that a 30 nm layer of silica has been successfully coated on the hollow nanosphere (Fig. 2d).
The as-prepared Gd2(CO3)3:Eu3+ HNSs showed no detectable XRD pattern (as shown in Fig. S2 ESI†), indicating that the product is amorphous. EDS analysis results were showed in Fig. 3. There were only two element peaks corresponding to Si and O in the curve a, no other impurity elements are found, that was consistent with the pure SiO2. Excepted the element of Si and O, the Gd and Eu was found in the curve b, which should be ascribed to the core–shell structured SiO2@Gd2(CO3)3:Eu. The element peak of Si was almost disappeared and the peak intensity of the Gd, Eu, C and O increased obviously for the hollow Gd2(CO3)3:Eu. Comparing the curve c and d, the peak of Si reappeared, that indicated the mesoporous silica was successfully coated onto the surface of the Gd2(CO3)3:Eu HNSs.
 | ||
| Fig. 3 EDS spectrum of the samples: SiO2 (a), core–shell-structured SiO2@Gd2(CO3)3:Eu (b), Gd2(CO3)3:Eu HNSs (c), Gd2(CO3)3:Eu@mSiO2 HNSs (d). | ||
IR spectroscopy was carried out to further examine the chemical compositions of the nanoparticle samples (Fig. 4). In the IR spectrum of the as-prepared SiO2 nanospheres, the absorption bands due to OH (3420 cm−1), H2O (1634 cm−1), Si–O–Si (νas, 1080 cm−1; νs, 796 cm−1), and Si–OH (νs, 950 cm−1) (where νas = asymmetric stretching, νs = symmetric stretching bending) were observed (Fig. 4a). In the Fig. 4b for the core–shell structured SiO2@Gd2(CO3)3:Eu, in addition to the strong absorption band of Si–O–Si (νas, 1080 cm−1), two absorption bands at 1509 cm−1 and 1406 cm−1 appeared, which were ascribed to the bands of νas O–C–O. This indicates the presence of the carbonate group. In the Fig. 4c for the hollow Gd2(CO3)3:Eu, the characteristic bands of Si–O–Si (νas, 1080 cm−1) and Si–OH (νs, 950 cm−1) almost disappeared, the absorption bands of O–H (3420 cm−1), O–C–O (1509 and 1406 cm−1), C–O (1086 cm−1) and p-CO3 (856 cm−1) were observed. For the Gd2(CO3)3:Eu@mSiO2 HNSs, the characteristic bands of Si–O–Si (νas, 1080 cm−1) and Si–OH (νs, 950 cm−1) appeared again (Fig. 4d).
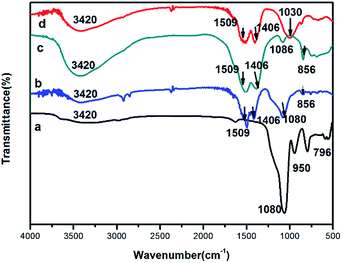 | ||
| Fig. 4 IR spectra of pure SiO2 NSs (a), SiO2@Gd2(CO3)3:Eu (b), Gd2(CO3)3:Eu HNSs (c), and Gd2(CO3)3:Eu@mSiO2 HNSs (d). | ||
The IR results indicate that the formation of Gd2(CO3)3:Eu@mSiO2 HNSs were subjected to four steps (Scheme 1). First, highly monodispersed SiO2 nanospheres were prepared by a modified Stőber method. Core–shell structured precursors were then produced by a homogeneous precipitation method using the silica nanospheres as the template and urea as the precipitation agent. Urea played a crucial role in the formation of the precursor shell on the surface of the silica nanospheres. The decomposition of urea slowly released precipitating anions (mainly OH− and CO32−) to the reaction systems at elevated temperature. And the precipitating anions dispersed on the surface of the silica sphere, so the Gd3+ and Eu3+are easily to adsorb on the silica core, resulting in the homogeneous precipitation of the uniform precursor nanoparticles (Gd2(CO3)3:Eu) coated on the surface of the silica template. The silica core was then removed with NaOH to produce Gd2(CO3)3:Eu HNSs. A mesoporous silica layer was then coated on the surfaces Gd2(CO3)3:Eu HNSs with CTAB as an organic template that was removed later to form Gd2(CO3)3:Eu@mSiO2 HNSs. Detailed experimental processes are given in the experimental section.
The specific surface area and porosity of Gd2(CO3)3:Eu@mSiO2 HNSs were determined by nitrogen adsorption method. The synthesised Gd2(CO3)3:Eu@mSiO2 HNSs showed a typical IV-type adsorption–desorption isotherm with a H1-hysteresis loop, suggesting its mesoporous structure (Fig. 5). The BET surface area and total pore volume of Gd2(CO3)3:Eu@mSiO2 HNSs were calculated as 143.17 m2 g−1 and 0.128 cm3 g−1, respectively. The pore-size distribution shows a narrow apex centered at 3.9 nm. These results evidently show the mesopore channels and large pore volume of the as-prepared Gd2(CO3)3:Eu@mSiO2 HNSs.
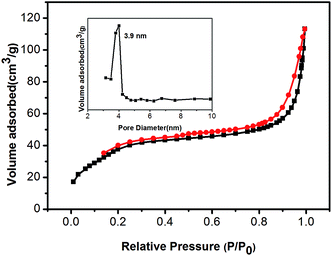 | ||
| Fig. 5 N2 adsorption–desorption isotherms and mesopore size distribution (the inset) of the synthesized Gd2(CO3)3:Eu@mSiO2. | ||
3.2 Optical properties
Fig. 6 shows the excitation and emission spectra of the as-prepared Gd2(CO3)3:Eu@mSiO2 HNSs. The broad excitation band with a maximum at 254 nm was attributed to the charge transfer transition from europium to oxygen (CTB of Eu → O) (Fig. 6a). The weak lines between 300 and 500 nm can be ascribed to the characteristic f–f transitions of Eu3+ within its 4f6 configuration. The relatively stronger peaks were found at 395 nm (7F0 → 5L6) and 466 nm (7F0 → 5D2). Fig. 6b shows emission spectrum of the as-prepared Gd2(CO3)3:Eu@mSiO2 HNSs under the UV excitation at 395 at room temperature. The emission spectrum is composed of 5D0 → 7FJ (J = 1, 2, 3, 4) transition lines of Eu3+ with the prominent peak of the 5D0 → 7F2 hypersensitive transition at 613 nm under the excitation at 395 nm. As the digital photo shown in the inset of Fig. 6b, the HNSs in EtOH exhibit bright red luminescence under UV excitation.3.3 Cell imaging
The application of Gd2(CO3)3:Eu@mSiO2 HNSs in the luminescence imaging of living cells was explored with NCI-H460 lung cancer cells. NCI-H460 lung cancer cells were incubated in a serum-free medium containing 100 μg mL−1 Gd2(CO3)3:Eu@mSiO2 HNSs at 37 °C for 4 h and examined under a confocal fluorescence microscopy. Red fluorescence was observed on the Gd2(CO3)3:Eu@mSiO2 HNSs treated cells under an excitation at 405 nm (Fig. 7a). The labeling of cells was confirmed with the phase contrast (Fig. 7b) and overlay (Fig. 7c) images. These results suggest that the Gd2(CO3)3:Eu@mSiO2 HNSs are able to enter living NCI-H460 lung cancer cells and can be used for cell imaging. | ||
| Fig. 7 The confocal fluorescence (a), phase contrast (b), and overlay (c) images of NCI-H460 lung cancer cells exposed to Gd2(CO3)3:Eu@mSiO2 HNSs for 4 h. | ||
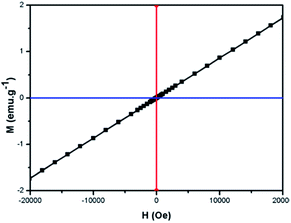
3.4 Magnetic and MRI studies
The magnetic property of the as-prepared Gd2(CO3)3:Eu@mSiO2 sample was determined with a magnetic property measurement system (MPMS XL-7). The plot of magnetization versus applied magnetic field for Gd2(CO3)3:Eu@mSiO2 HNSs was shown in Fig. 8. It showed the magnetization linearly increased with the increase of the applied magnetic field strength, indicating that the as prepared Gd2(CO3)3:Eu@mSiO2 HNSs possessed paramagnetism due to the seven unpaired inner 4f electrons of Gd3+ ions and is a promising candidate of MRI contrast agent. Fig. 9 shows the respective T1-weighted images with a series of different Gd3+ concentrations (0.0, 0.2, 0.4, 0.5, 1.0 mM). The T1-weighted images gradually became brighter, which accounts for positive enhancement of the effect on T1-weighted sequences. The relaxation (r1) value of the sample calculated from the slope of the concentration dependent relaxation T1−1, was estimated to be 1.15 mM−1 S−1. All of these results reveals the potential use of the Gd2(CO3)3:Eu@mSiO2 HNSs as an effective T1 contrast agent.3.5 Drug adsorption and release properties
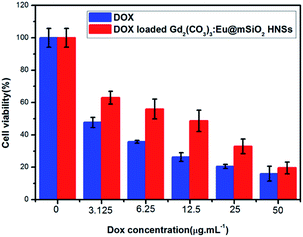
DOX was selected as a model drug to determine the drug storage and release properties of DOX loaded Gd2(CO3)3:Eu@mSiO2 HNSs. The drug loading amount was calculated to be 93.3 μg mg−1 Gd2(CO3)3:Eu@mSiO2 HNSs. The drug releasing behavior of DOX-loaded particles was evaluated in PBS of two different pH values (7.4 and 5.8). Fig. 10 shows the cumulative DOX release profile of the DOX loaded Gd2(CO3)3:Eu@mSiO2 in PBS as a function of release time. The drug release rate of the DOX loaded Gd2(CO3)3:Eu@mSiO2 was obviously pH dependent. Only 38.5% is released after 24 h when pH = 7.4. The release amount reached to 54.8% with the pH value decreased to 5.8. This trend may be explained by the increasing hydrophilicity and high solubility of DOX in the low pH.33 Considering the different pH value in blood (7.4), especially cancer tissue is acidic extracellular (5.8–7.2), the pH-sensitive drug release property is beneficial to targeting cancer tissues and reducing toxic side effect for normal tissues.3.6 In vitro cytotoxicity
In vitro cytotoxicity effects of the DOX and DOX loaded Gd2(CO3)3:Eu@mSiO2 HNSs were tested on NCI-H460 lung cancer cells via MTT assay. The concentrations of the free DOX and the DOX content in the DOX loaded Gd2(CO3)3:Eu@mSiO2 HNSs were 0, 3.125, 6.25, 12.5, 25, 50 μg mL−1, respectively. As shown in Fig. 11, both free DOX and DOX loaded Gd2(CO3)3:Eu@mSiO2 HNSs exhibited an increasing inhibition against NCI-H460 cells with the concentration increasing. At lower concentration, free DOX shows higher cytotoxicity than the DOX loaded Gd2(CO3)3:Eu@mSiO2 HNSs, but when the concentration increased to 25 μg mL−1 similar cytotoxicity was founded. That can be ascribled to the sustainable release behavior of the DOX loaded Gd2(CO3)3:Eu@mSiO2 HNSs. In addition, MTT assay (Fig. S4, ESI†) demonstrates the satisfactory biocompatibility of the Gd2(CO3)3:Eu@mSiO2 HNSs, indicating that the nontoxic property of the sample. Based on these results, the Gd2(CO3)3:Eu@mSiO2 HNSs can potentially be used as anti-cancer drugs carrier and enhance the anti-cancer drug delivery efficacy.4 Conclusions
In summary, we have successfully prepared the well-dispersed mesoporous silica coated Gd2(CO3)3:Eu HNSs by a template-directed method with silica NSs as the template. It showed both excellent fluorescence and magnetic properties and thus could be used as a dual-imaging agent for optical/MR imaging. In addition, Gd2(CO3)3:Eu@mSiO2 HNSs showed pH-dependent DOX release property, indicating its potential application in antitumor drug delivery. The efficient optical and MR imaging capabilities, as well as the hollow structure of Gd2(CO3)3:Eu@mSiO2 HNSs make it a promising platform for simultaneous bioimaging and drug delivery.Acknowledgements
This work was supported by the NSFC (No. 21462019), NCET (11-1000), Bureau of Science & Technology of Jiangxi Province (20143ACB20012), and Bureau of Science & Technology of Nanchang City for financial support.Notes and references
- L. Cheng, K. Yang, Y. Li, J. Chen, C. Wang, M. Shao, S. T. Lee and Z. Liu, Angew. Chem., Int. Ed., 2011, 50, 7385–7390 CrossRef CAS PubMed.
- H. Gong, Z. L. Dong, Y. M. Liu, S. N. Yin, L. Cheng, W. Y. Xi, J. Xiang, K. Liu, Y. G. Li and Z. Liu, Adv. Funct. Mater., 2014, 24, 6413 CrossRef CAS.
- L. J. Ao, B. Wang, P. Liu, L. Huang, C. X. Yue, D. Y. Gao, C. L. Wu and W. Su, Nanoscale, 2014, 6, 10710–10716 RSC.
- T. Kim, E. Momin, J. Choi, K. Yuan, H. Zaidi, J. Kim, M. Park, N. Lee, M. T. McMahon, A. Quinones-Hinojosa, J. W. M. Bulte, T. Hyeon and A. A. Gilad, J. Am. Chem. Soc., 2011, 133, 2955–2961 CrossRef CAS PubMed.
- J. Zhang, Y. H. Wang, Z. G. Xu, H. X. Zhang, P. Y. Dong, L. N. Guo, F. H. Li, S. Y. Xin and W. Zeng, J. Mater. Chem. B, 2013, 1, 330–338 RSC.
- I. F. Li, C. H. Su, H. S. Sheu, H. C. Chiu, Y. W. Lo, T. W. Lin, J. H. Chen and C. S. Yeh, Adv. Funct. Mater., 2008, 18, 766–776 CrossRef CAS.
- Y. H. Han, S. L. Gai, P. A. Ma, L. Z. Wang, M. L. Zhang, S. H. Huang and P. P. Yang, Inorg. Chem., 2013, 52, 9184–9191 CrossRef CAS PubMed.
- W. R. Zhao, H. R. Chen, Y. S. Li, L. Li, M. D. Lang and J. L. Shi, Adv. Funct. Mater., 2008, 18, 2780–2788 CrossRef CAS.
- G. X. Yang, S. L. Gai, F. Y. Qu and P. P. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 5788–5796 Search PubMed.
- T. Kim, N. Lee, Y. I. Park, J. W. Kim, J. Y. Kim, E. Y. Lee, M. Y. Yi, B.-G. Kim, T. W. Hyeon, T. Y. Yu and H. B. Na, RSC Adv., 2014, 4, 45687–45695 RSC.
- D. M. Yang, P. A. Ma, Z. Y. Hou, Z. Y. Cheng, C. X. Li and J. Lin, Chem. Soc. Rev., 2015, 44, 1416–1448 RSC.
- F. He, P. P. Yang, D. Wang, C. X. Li, N. Niu, S. L. Gai and M. L. Zhang, Langmuir, 2011, 27, 5616–5623 CrossRef CAS PubMed.
- H. P. Singh, S. Mitra and R. K. Sharma, RSC Adv., 2014, 4, 61028–61035 RSC.
- Z. H. Xu, Y. Cao, C. X. Li, P. A. Ma, X. F. Zhai, S. S. Huang, X. J. Kang, M. M. Shang, D. M. Yang, Y. L. Dai and J. Lin, J. Mater. Chem., 2011, 21, 3686–3694 RSC.
- M. L. Debasu, A. Duarte, S. L. C. Pinho, F. G. C. Geraldes, L. D. Carlos and J. Rocha, Nanoscale, 2012, 4, 5154–5162 RSC.
- Z. G. Yi, W. Lu, C. Qian, T. M. Zeng, L. Z. Yin, H. B. Wang, L. Rao, H. R. Liu and S. J. Zeng, Biomater. Sci., 2014, 2, 1404–1411 RSC.
- W. Ren, G. Tian, L. J. Zhou, W. Y. Yin, L. Yan and S. Jin, Nanoscale, 2012, 4, 3754–3760 RSC.
- Y. L. Deng, H. Wang, W. Gu, S. Li, N. Xiao, C. Shao, Q. Y. Xu and L. Ye, J. Mater. Chem. B, 2014, 2, 1521–1529 RSC.
- M. He, P. Huang, C. L. Zhang, H. Y. Hu, C. C. Bao, G. Gao, R. Heand and D. X. Cui, Adv. Funct. Mater., 2011, 21, 4470–4477 CrossRef CAS.
- S. Majeed and S. A. Shivashankar, J. Mater. Chem. B, 2014, 2, 5585–5593 RSC.
- Y. Gao, Q. Zhao, Q. Fang and Z. H. Xu, Dalton Trans., 2013, 42, 11082–11091 RSC.
- S. P. Fricker, Chem. Soc. Rev., 2006, 35, 524–533 RSC.
- L. J. Zhou, W. Y. Yin, W. L. Ren, Z. J. Gu, L. Wei and S. Jin, New J. Chem., 2012, 36, 2599–2606 RSC.
- Y. L. Wu, X. Z. Xu, Q. Tang and Y. X. Li, Nanotechnology, 2012, 23, 205103–225109 CrossRef PubMed.
- Y. Gao, M. M. Fan, Q. H. Fang and F. Yang, New J. Chem., 2014, 38, 146–154 RSC.
- Q. Ji, J. P. Hill and K. Ariga, J. Mater. Chem. A, 2013, 1, 3600–3606 RSC.
- S. H. Wu, Y. Hung and C. Y. Mou, Chem. Mater., 2013, 25(3), 352–364 CrossRef CAS.
- K. Y. J. Son, H.-J. Yoon, J.-H. Kim, W.-D. Jang, Y. Lee and W.-G. Koh, Angew. Chem., Int. Ed., 2011, 50, 11968–11971 CrossRef CAS PubMed.
- S. H. Tang, X. Q. Huang, X. L. Chen and N. F. Zheng, Adv. Funct. Mater., 2010, 20, 2442–2447 CrossRef CAS.
- R. W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- F. Y. Liu, X. X. He, L. Liu, H. P. You, H. M. Zhang and Z. X. Wang, Biomaterials, 2013, 34, 5218–5225 CrossRef CAS PubMed.
- S. L. Gai, P. P. Yang, C. X. Li, W. X. Wang, Y. L. Dai, N. Niu and J. Lin, Adv. Funct. Mater., 2010, 20, 1166–1172 CrossRef CAS.
- Y. Wu, D. M. Yang, X. J. Kang, P. A. Ma, S. S. Huang, Y. Zhang, C. X. Li and J. Lin, Dalton Trans., 2013, 42(27), 9852–9861 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07444h |
| This journal is © The Royal Society of Chemistry 2016 |