A novel strategy to synthesize bimetallic Pt–Ag particles with tunable nanostructures and their superior electrocatalytic activities toward the oxygen reduction reaction
Min Liu,
Fangze Chi,
Jingjun Liu*,
Ye Song and
Feng Wang*
State Key Laboratory of Chemical Resource Engineering, Beijing Key Laboratory of Electrochemical Process and Technology for Materials, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: liujingjun@mail.buct.edu.cn; wangf@mail.buct.edu.cn; Fax: +86 10 64411301; Tel: +86 10 64411301
First published on 22nd June 2016
Abstract
The ability to precisely control the nanoscale phase structure of bimetallic catalysts is required to achieve a synergistic effect between two metals for the oxygen reduction reaction (ORR). In this work, we synthesized Pt–Ag bimetallic nanoparticles (NPs) with Ag@Pt core–shell, highly alloyed solid and hollow nanostructures respectively, via a galvanic replacement reaction by modifying H2PtCl6 concentration in an aqueous solution containing homemade Ag NPs as the sacrificial templates. The nanophase and corresponding electronic structures of the synthesized Pt–Ag NPs were characterized by transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy. The formation of these Pt–Ag NPs with different nanophase structures is closely ascribed to a defect-induced Kirkendall effect that involves the accelerated interdiffusion of Ag and Pt atoms, triggered by the high density of defects along the Ag NP surface generated by the galvanic replacement reaction. The nanophase structure-dependent electrocatalytic activity of three Pt–Ag bimetallic NPs was determined in 0.5 M H2SO4 solution by using a rotating disk electrode (RDE). The results showed that the core–shell and hollow alloy NPs exhibit excellent ORR activity in acidic solution, which is remarkably higher than that of the commercial Pt/C (E-TEK). The physical origin of the enhancement in the ORR activity can be explained by a mutual ligand effect, raised by the substantial electronic transfer between Pt and Ag at the atomic level, which results from the downshift of the d-band center for Pt and the increased number of the unpaired electrons for Ag in these bimetallic catalysts. Thus two factors achieve a synergistic effect that dominates the remarkably improved electrocatalytic activity for the ORR.
1. Introduction
The oxygen reduction reaction (ORR) has long been considered as one of the most important electrochemical processes in advanced electrochemical energy storage and conversion devices including various proton exchange membrane fuel cells and metal–air batteries.1 Platinum nanoparticles with diameters of 2–5 nm on carbon (Pt/C) are currently considered as the best catalysts for the ORR in both acidic and alkaline environments.2 However, the high cost and degradation of Pt with increased operation time severely restrict the practical application of Pt catalysts.3,4 Therefore, the development of Pt-based alloys as efficient and cheap next-generation catalysts for the ORR is urgent for the application of the ORR in these fields. Many studies have suggested that Pt alloyed with a secondary metal (M = Co, Ni, Fe, Ag, Cu) may not only help to reduce the usage of Pt, but also allow the individual functional components to work synergistically to improve the catalytic performance or to obtain additional performance that may not be achieved by a monometallic catalyst such as Pt; thus, this approach promises a much more flexible catalyst design.5,6Among these bimetallic catalysts, Pt–Ag systems have recently attracted attention owing to their excellent ORR activity and stability.7–9 Some works have shown that Pt–Ag bimetallic catalysts exhibit high electrocatalytic activity towards ORR that is superior to that of Pt/C catalyst.6 The physical origin of the enhanced activity for the bimetallic catalysts still remains unclear, but it is generally accepted that the ORR activity of the catalyst substantially depends on the geometric (Pt–Pt interatomic distances) and electronic (ligand) factors. There have been several possibilities associated with the favorable ORR kinetics on Pt–Ag bimetallic systems. On the one hand, Pt alloyed with Ag atoms would result in the downshift of the d-band center for Pt component relative to the Fermi level, which can weaken the strongly chemical adsorption of oxygen/oxides/anions (the d-band centre is too close to the Fermi level), which contributes to enhance ORR activity, relative to that of pure Pt.10,11 This assumption has been evidenced by the downshift of the d-band center for Pt in the synthesized Pt–Ag alloy particles.12 On the other hand, the presence of Ag component in this bimetallic system can strengthen the chemical adsorption strength of active oxygen during the ORR due to the upshift of the d-band center for Ag, which leading to more electrochemically active sites available for ORR over Ag. Hence, achieving synergy between Pt and Ag components is a feasible way to develop highly active Pt-based catalysts in various environments, especially in acid environment that is more important than base environment because of their potential application in proton exchange membrane fuel cell.
Although the influence of surface electronic structure of Pt-based catalysts on the ORR processes is still debated, it is believed that ORR kinetics strongly depends on nanophase structures of the catalysts that dominate the interaction energy between oxygen and the metals such as Pt, Au, Ag and Pd.13–15 Thus, the synergistic effects can be achieved and eventually remarkably enhanced by manipulating the smart nanophase structures of the Pt–Ag bimetallic catalysts, as evidenced by the previous investigators.16,17 However, for noble metals or bimetallic alloys,18–20 the precise control of their morphology and nanophase still remains a serious challenge, because simple and environmentally friendly synthesis of them is difficult in an aqueous solution without additives.20 But, this control is required to develop highly active materials with designed catalytic performance by tuning their electronic and geometric effects.
Herein, we reported a novel and facile synthesis route to fabricate platinum–silver bimetallic nanoparticles featuring tunable nanostructures via a modified galvanic replacement reaction in an aqueous solution containing chloroplatinic acid and homemade silver particles supported on carbon as sacrificial templates. Utilizing this synthesis strategy, a series of Pt–Ag bimetallic nanostructures, such as Ag@Pt core–shell, highly alloyed solid and hollow nanoparticles, were fabricated by simple regulating concentrations of H2PtCl6 in the aqueous solution. The nanostructure-dependent electrocatalytic activity in ORR over these bimetallic catalysts were characterized in an acidic solution. Moreover, X-ray photoelectron spectroscopy (XPS) was carried out for these synthesized Pt–Ag nanostructures in order to elucidate the intrinsic correlation between electronic states of these nanostructures and their relevant ORR activity. This information is important for the understanding of the physical origin of the enhanced ORR kinetics on the Pt-based bimetallic catalysts.
2. Experimental
The carbon-supported Pt–Ag bimetallic nanoparticles (NPs) with Ag@Pt core–shell, solid and hollow alloy structures have been fabricated via a galvanic replacement reaction between chloroplatinic acid and carbon-supported silver nanoparticles as sacrificial templates in an aqueous solutions. Initially, we synthesized the carbon-supported silver nanoparticles (Ag/C) with diameter of about 10 nm by using polyol method at 120 °C. A typical preparation procedure for the Ag/C is as following. Carbon black (Vulcan XC-72, CABOT Corporation) as support was subjected to the surface chemical modification in nitric acid (HNO3, concentration: 14.6 M) at 140 °C for 5 h, followed by washing to neutral with centrifuge and drying in vacuum. Then, the treated carbon black (10 mg) was added to ethylene glycol (C2H6O2, 10 mL) and dispersed by sonication to form a suspension of carbon black and ethylene glycol. In parallel, silver nitrate (AgNO3, 18.5 mL, 0.1 M) was added to ammonia (NH3·H2O, 55.5 mL, 0.1 M) to form silver ammonia solution. Finally, the resulting silver ammonia solution (3.7 mL) was mixed with the previously prepared suspension of carbon black and ethylene glycol, followed by the addition of NaOH (4 mL, 1 M) and stirring at 120 °C for 1 h, then cooled to room temperature.A typical preparation procedure for the Pt–Ag bimetallic NPs is as following. A certain amount of H2PtCl6 (0.01 M) and aqueous sodium citrate (4.6 mL, 0.04 M) were successively dropped to the reaction solution containing Ag/C sample and the galvanic reaction would occurred and lasted under magnetic stirring for 2 hour to obtain Pt–Ag bimetallic NPs at 90 °C. The different Pt–Ag bimetallic NPs with core–shell, alloyed solid and hollow nanostructures were easily achieved by adding 2.3 mL, 4.6 mL, 9.2 mL of 0.01 M H2PtCl6 aqueous in the reaction solution, respectively. Then, the synthesized bimetallic NPs were simply centrifuged, then washed with deionized water and ethanol sequentially, followed dry in a vacuum at 80 °C for 8 h. The compositions of the prepared bimetallic samples were assessed by inductively coupled plasma mass spectrometry (ICP-MS) measurements. For the Ag@Pt core–shell nanoparticles, the determined atomic ratios of platinum to silver is 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80; for the solid and hollow-type Pt–Ag alloys, the atomic ratios of platinum to silver of each sample is 40
80; for the solid and hollow-type Pt–Ag alloys, the atomic ratios of platinum to silver of each sample is 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 and 40
65 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57, respectively. The metal (Pt + Ag) loading is 40 wt% relative to the carbon for these synthesized Pt–Ag bimetallic catalysts.
57, respectively. The metal (Pt + Ag) loading is 40 wt% relative to the carbon for these synthesized Pt–Ag bimetallic catalysts.
The morphologies of the prepared Pt–Ag bimetallic NPs were observed by high-resolution transmission electron microscopy (HRTEM, JEM-2100F, JEOL). The crystalline structures of these Pt–Ag bimetallic samples were determined by X-ray powder diffractometer (XRD, RINT 2000 V/PC, Japan) using Cu Kα radiation operating at 40 kV and 200 mA under a scanning rate of 0.02 deg per s in the angle (2θ) range of 10° to 90°. To study electronic structures of the Pt–Ag bimetallic NPs, X-ray photoelectron spectra (XPS, PHI Quantera SXM with monochromatized Al Kα radiation, 15 kV and 25 W, and an energy resolution of 0.1 eV) were obtained from a monochromator (Al Kα radiation) and all peak positions were normalized to the binding energy of C 1s (284.8 eV). To confirm the formation of Ag@Pt core–shell NPs, the Rhodamine 6G adsorbed Pt–Ag bimetallic NPs were characterized by Raman scattering measurements (LabRam HR800 Raman spectrometer) using 633 nm laser with the range of 2000 to 200 cm−1.
The electrochemical measurements of the synthesized bimetallic samples, including cyclic voltammetry (CV) and a rotating disk electrode (RDE) were conducted using Autolab PGSTAT302N (Metrohm, Swiss Confederation) and a rotating disk electrode device (PINE, USA) respectively. The measurements were performed in a typical three-electrode system at room temperature with a platinum wire as counter electrode and a saturated calomel electrode (SCE) as reference electrode. The disk potential of RDE was scanned at 5 mV s−1. The working electrodes were prepared by depositing a thin layer of catalyst ink onto a glassy carbon (GC, area of 0.247 cm2 for RDE and area of 0.071 cm2 for CV). The catalyst ink was prepared by dispersing 10 mg of the catalyst in a mixture of 1 mL ethanol and 1 mL water, followed by addition of 100 μL of film-forming reagent i.e., Nafion aqueous solution (5 wt%, DUPONT, USA). The suspension was sonicated for 30 min to form a homogeneous ink, then pipetted onto the surface of the GC electrode and dried at room temperature. For the bimetallic Pt–Ag catalysts, the metal platinum loading on a RDE was 0.064 mg cm−2 (Agcore–Ptshell NPs), 0.105 mg cm−2 (solid alloy NPs) and 0.168 mg cm−2 (hollow alloy NPs). During the electrochemical measurements, a commercial Pt/C catalyst (40% Pt with particle size of 3.2 nm on Vulcan XC-72 carbon support, E-TEK, Somerset, NJ) can be taken as a reference material. The Pt loading on the RDE was 0.109 mg cm−2 for the Pt/C.
3. Results and discussion
3.1 Fabrication of Pt–Ag bimetallic nanoparticles with different nanostructures
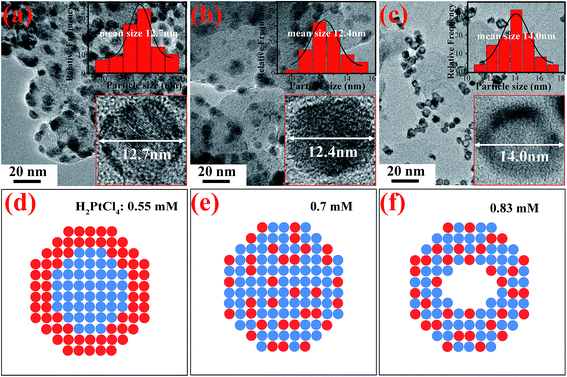
The ability to control nanoscale phase structures of the bimetallic Pt-based nanoparticles (NPs) provides a means for exploring bimetallic materials as advanced ORR catalysts. Therefore, we have synthesized three Pt–Ag bimetallic NPs with different nanostructures, by simply regulating concentrations of H2PtCl6 in aqueous solutions containing homemade carbon-supported Ag NPs as the sacrificial templates via a galvanic replacement reaction. Fig. 1 presents TEM images of the synthesized Pt–Ag bimetallic NPs with different nanostructures, respectively. From Fig. 1a–c, the larger gray particles are carbon black supports, and the dark and spherical small particles anchored on the carbon surfaces should be Pt–Ag bimetallic NPs, since heavy metals usually present strong contrast against the background. It can be noted that these bimetallic NPs are homogeneously and separately deposited over the carbon surface, and no aggregated particles are observed. Based on the associated particle size histograms in these HRTEM images in Fig. 1a–c (the insets), the average particle sizes of these Pt–Ag bimetallic NPs fabricated at different H2PtCl6 concentrations are about 12.7, 12.4 and 14.0 nm, respectively. Moreover, we have observed from Fig. 1a–c that the morphologies of the three NPs are obviously different, depended on the H2PtCl6 concentrations in solutions. As H2PtCl6 concentration is fixed at 0.55 mM, the morphology of the prepared bimetallic Pt–Ag NPs features a core–shell-like structure, as shown in Fig. 1a (inset). It can be seen that the core metal is silver (gray) and covered by Pt (dark) because different colors indicate the presence of different metals, where the dark spots refer to Pt and gray to Ag in the core–shell NPs, since the heavy metal Pt usually present strong contrast against Ag.21Along with further increasing H2PtCl6 concentrations under the same reaction conditions, the NPs prepared at 0.70 mM display a solid-type alloy shown in Fig. 1b (inset), but the NPs prepared at 0.83 mM show a hollow-type alloy, as shown in Fig. 1c (inset). These outcomes strongly imply the significant concentration-effect on their nanostructures for the Pt–Ag NPs fabricated via the galvanic replacement reaction. Therefore, by taking advantage of this galvanic replacement, the Pt–Ag bimetallic catalysts with core–shell (Fig. 1d), solid (Fig. 1e) and hollow alloy (Fig. 1f) nanostructures can be fabricated by simply tuning concentrations of H2PtCl6 in aqueous solutions. According to previous works in the literature,22 the nanoscale phase structure plays a key role in determining the catalytic properties of bimetallic catalysts. Regarding of the Pt–Ag bimetallic NPs fabricated at 0.70, 0.83 mM respectively, however, all the recorded diffraction peaks in their XRD patterns position are between the reflections of pure Ag and pure Pt shown in Fig. 2, illustrating the formation of single-phase alloyed Pt–Ag NPs.
 | ||
| Fig. 2 XRD patterns of the synthesized Agcore@Ptshell nanoparticles, solid and hollow Pt–Ag alloy nanoparticles, respectively. | ||
To further investigate the nanophase structure and alloying evolution of this bimetallic system, therefore, XRD measurements were carried out for these three Pt–Ag NPs and the obtained results are shown in Fig. 2. As observed, the first peak located at about 24.8° in all the XRD patterns is ascribed to the face-to-face stacking of C (002) crystalline plane of the carbon support. Similar to the pure Ag/C sample, the NPs fabricated at 0.55 mM H2PtCl6 exhibit five distinct diffraction peaks located at about 38.0°, 44.2°, 64.4°, 77.3° and 81.5°, which are indexed to the (111), (200), (220), (311) and (222) crystal facets of metallic Ag with a face-centered cubic (fcc) structure (JCPDS card file 04-0783). The other five peaks for the sample recorded at about 40°, 46°, 67°, 81° and 85.7° are assigned to the (111), (200), (220), (311) and (222) facets of the face-centered cubic (fcc) crystal structure of Pt (JCPDS card file 04-0802), indicating that the NPs are composed of both metallic silver and platinum phases. No Pt–Ag alloy phase is observed in this case.
Regarding of the Pt–Ag bimetallic NPs fabricated at 0.70, 0.83 mM respectively, however, all the recorded diffraction peaks in their XRD patterns position are between the reflections of pure Ag and pure Pt shown in Fig. 2, illustrating the formation of single-phase alloyed Pt–Ag NPs. The alloying degree of these two alloys is very high due to their remarkably shifted diffraction peaks relative to those of two pure metal, implying that the alloy degree of these alloys is at the atomic level. Therefore, we concluded that the design of the nanoscale phase structure for Pt–Ag systems can be achieved by way of the simple galvanic replacement reaction. The control of the bimetallic spatial arrangement of Pt and Ag atoms in their bimetallic NPs for advanced catalysts is essential because it provides a promising way for establishing the correlation between the nanoscale phase structure and the electrocatalytic activity for ORR.22,23 However, the exact formation mechanism of these Pt–Ag bimetallic NPs with different structures remains a puzzle by now.
3.2 Formation mechanism of Pt–Ag bimetallic NPs with different nanostructures
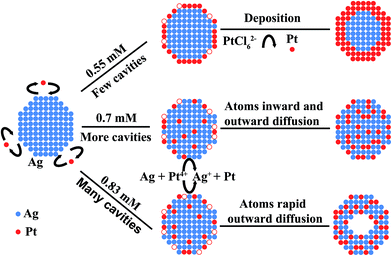
A possible formation mechanism of these Pt–Ag bimetallic NPs featuring different nanostructures, such as core–shell, solid-type and hollow-type alloyed, may be attributed to the rate of the controlled rate of the galvanic replacement reaction between H2PtCl6 and Ag NPs, dependent on the H2PtCl6 concentrations, as shown in Fig. 3. The galvanic replacement reaction (eqn (1)) is feasible because the standard reduction potentials of PtCl62−/PtCl42− and PtCl42−/Pt pairs (0.68 V and 0.73 V respectively, versus SHE) are much more positive than that of Ag/AgCl/Cl− redox couple (0.222 V versus SHE), as shown in eqn (2)–(4). However, the rate of the galvanic replacement reaction between H2PtCl6 and Ag strongly depends on the activity or effective concentration of Cl− ions in aqueous solution. At a low H2PtCl6 concentration, the rate of the replacement reaction should be very slow due to the low Cl− ion concentration released from the dilute PtCl62− concentration.24,25| 4Ag(s) + PtCl62−(aq) → Pt(s) + 4AgCl(s) + 2Cl−(aq) | (1) |
| PtCl62−(aq) + 2e− → PtCl42−(aq) + 2Cl−(aq), E0 = +0.68 V vs. SHE | (2) |
| PtCl42−(aq) + 2e− → Pt(s) + 4Cl−(aq), E0 = +0.73 V vs. SHE | (3) |
| Ag(s) + Cl−(aq) → AgCl(s) + e−, E0 = +0.222 V vs. SHE | (4) |
 | ||
| Fig. 3 Schematic diagram of the formation of three Pt–Ag bimetallic nanoparticles with different nanostructures including Agcore@Ptshell nanoparticles, solid and hollow Pt–Ag alloyed nanoparticles. | ||
Therefore, as H2PtCl6 concentration is lower than 0.55 mM, the Pt–Ag alloyed NPs cannot form due to the sluggish replacement reaction in this case. But, Ag@Pt core–shell NPs have been fabricated at 0.55 mM H2PtCl6, as evidenced in Fig. 1a and 2. The formation of these Ag@Pt core–shell NPs at this stage is attributed to the chemical reduction of H2PtCl6 by the added sodium citrate as stable and reducing agent in the reaction solution. As silver NPs are added into the synthesis system, the reduced Pt atoms by sodium citrate would preferentially deposit onto the surfaces of the silver NPs rather than cover the carbon surface since the chemical interaction between metals is stronger than that between metals and carbon, which leads to the growth of Pt on Ag surface.26 In this way, the Ag NPs act as the cores, and the reduced Pt covering the Ag cores acts as the shells, forming the Ag@Pt core–shell NPs. To verify the core–shell NPs with a well-defined structure, the selective adsorption of Rhodamine 6G on the synthesized Ag@Pt core–shell NPs was characterized by surface-enhanced Raman scattering (SERS). As depicted in Fig. 4a, no any signal of surface-enhanced scattering for the core–shell NPs has been observed from the Rhodamine 6G species. The similar results were observed for the pure Pt NPs except two prominent peaks at ∼1585 cm−1 and at ∼1351 cm−1 corresponding to the G and D bands of carbon matrix, as depicted in Fig. 4b. On the contrary, the recorded Raman spectrum of the Ag NPs adsorbed by the same Rhodamine 6G species shows several very strong resonance light-scattering peaks shown in Fig. 4c. These results further evidenced the formation of a well-defined core–shell structure, where Ag cores were fully covered with a Pt shell.
At a given higher H2PtCl6 concentration (0.70 mM), however, solid Pt–Ag alloy NPs can be prepared, as depicted in Fig. 1b. At this stage, the galvanic replacement of Ag with PtCl62− takes place because of the higher H2PtCl6 concentration that enables the electrochemical reaction (eqn (1)) to proceed faster than the low H2PtCl6 concentration, which contributes to the formation of the solid-type alloy NPs (Fig. 1b). The analysis of XRD results indicates that the electrochemical reaction has indeed occurred due to the appearance of diffraction peaks of solid AgCl, as shown in Fig. 4d. The formation of the Pt–Ag alloy NPs may be ascribed to an interdiffusion of Ag and Pt atoms that involves the nanoscale Kirkendall effect or defect-induced Kirkendall diffusion processes.27 In our case, as the concentrated H2PtCl6 is injected to the solution, the replacement reaction would start from twinning boundaries and stacking faults, instead of stable crystalline faces of Ag NPs; the electrochemically reduced Pt atoms should deposit non-epitaxially onto the surface of the Ag NPs because the lattice mismatch between Pt and Ag is 4.15%. In this case, large strain energy operates and non-epitaxial growth takes place. The non-epitaxial growth of Pt is associated with the surface free energy of the Ag template because the removing of four silver atoms can only generate one reduced platinum atom in reference to eqn (1), which leads to a large number of vacancy defects along the Ag NP surface during the replacement reaction. The presence of the high density of defects, raised by the galvanic replacement, has been confirmed by previous work.28 Thus, these defect sites can not only enhance the surface free energy of the Ag NPs but also act as diffusion channels to facilitate the outward diffusion of Ag atoms from the inside to the surface, following the inward migration of Pt atoms from the surface into inside to form the hollow structure, based on the Kirkendall effect.29,30 Such defect-accelerated interdiffusion of Ag and Pt atoms over the entire NPs is responsible for the formation of the homogeneous solid-solution alloy nanoparticles at the atomic level.
However, with further increasing the H2PtCl6 concentration from 0.70 to 0.83 mM, a transition from solid-type to hollow-type structure for the Pt–Ag alloy NPs was observed in Fig. 1c. At this stage (0.83 mM), the formation of the hollow Pt–Ag alloy NPs can be explained by the very fast replacement reaction between Ag and PtCl62− due to the high Cl− ion concentration released from PtCl62−, compared to the samples fabricated at lower H2PtCl6 concentrations. The fast replacement reaction consumes most of the Ag atoms at the surfaces of the template. As a result, a lot of defect sites or lattice vacancies would be generated shortly on the surface of Ag NPs. In this case, the increased galvanic replacement rate would, in turn, trigger the accelerated outward diffusion of Ag atoms from the inside to the surface to minimize the high surface energy. As the outward diffusion of Ag atoms is significantly faster than the inward diffusion of the reduced Pt atoms, an outward Ag atoms flux from the inside to the surface of each NP occurred, accompanying the inward flux of vacancies accompanies to balance the diffusivity difference.29–32 With increased reaction time, the interior in the core NPs would results in the supersaturation of lattice vacancies inside the NPs, which would condense to form voids in each NP.31 Subsequently, these voids inside the NPs tend to grow and then collapse in the center, eventually leaving Pt–Ag hollow nanostructures behind. Therefore, it is concluded that the formation of these Pt–Ag bimetallic NPs with different structures, such as core–shell, solid-type and hollow-type structures, is ascribed to the replacement reaction rates of H2PtCl6 with Ag, dependent on the H2PtCl6 concentrations in the solution. Although more evidence is needed to support this exact process, the fabrication of these bimetallic NPs with controlled structures via the simply modified replace reaction will provide new exciting opportunities to tune their optical, electrical, and electrocatalytic properties.
3.3 Electrocatalytic activity for ORR on these Pt–Ag bimetallic nanostructures
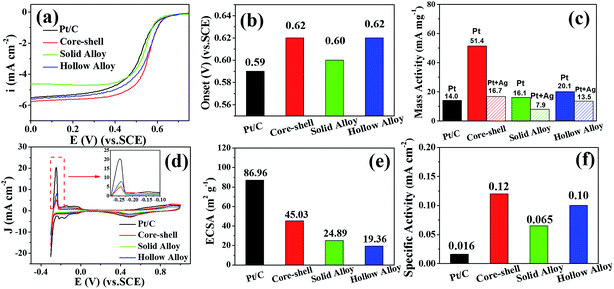
We benchmarked nanostructure-dependent electrocatalytic activity toward the ORR over these Pt–Ag bimetallic NPs featuring different nanostructures, such as core–shell, solid-type and hollow-type alloy, in an acidic solution at room temperature. A commercial Pt/C catalyst (40 wt% Pt with particle size of 3.2 nm on Vulcan XC-72 carbon support, E-TEK, Somerset, NJ) can be taken as a reference material. Fig. 5a shows the electrocatalytic trends for ORR on these Pt-based catalysts, evaluated by using a rotating disk electrode (RDE) at a fixed rotation rate of 1600 rpm in an oxygen-saturated 0.5 M H2SO4 solution. As observed in Fig. 5b, the onset potential, measured from different catalysts, increases in the order: Pt/C < solid alloy < hollow alloy < Ag@Pt core–shell, with the onset potential values of 0.59 V, 0.60 V, 0.62 V and 0.62 V versus a saturated calomel electrode (SCE), respectively. Among all the tested Pt-based catalysts, the Ag@Pt core–shell and the hollow alloy catalyst display the highest electrocatalytic activity for the ORR. In contrast, solid-type Pt–Ag alloy exhibits slightly enhanced ORR activity with respect to the commercial Pt/C, revealing effect of the nanophase structures on the electro-catalytic activity. To deeply explore the ORR kinetics over these catalysts, the kinetic current densities (Jk) were calculated from the ORR polarization curves by mass-transport correction and normalized to the loading amount of two metals in order to compare the mass-specific activities of these catalysts. At a given potential of 0.6 V versus SCE, the obtained mass-specific activities for these different catalysts are shown in Fig. 5c. Compared with that obtained by the state-of-the-art Pt/C (14.0 mA mg−1Pt), the Ag@Pt core–shell catalyst exhibits the remarkably enhanced mass-specific activities (16.7 mA mg−1Pt+Ag) on the basis of the total mass of Ag and Pt, whereas the hollow-type alloy also displays the improved mass-specific activity (13.5 mA mg−1Pt+Ag). However, the solid-type alloy exhibited a small mass-specific activity (7.9 mA mg−1Pt+Ag) toward the ORR. If the Pt mass is only taken into account, the mass-specific activity of three Pt–Ag bimetallic catalysts is much higher than that of the Pt/C catalyst, as shown in Fig. 5c. On the basis of the Pt mass, the obtained mass-specific activities for the Ag@Pt core–shell, Pt–Ag hollow alloy, and solid alloy are 51.4, 20.1 and 16.1 mA mg−1Pt, which are 3.7, 1.4 and 1.2 times greater than that of the stated Pt/C (14.0 mA mg−1Pt), respectively. Together with the oxygen reduction onset potential and mass-specific activity measurements, it is believed that Ag@Pt core–shell NPs and Pt–Ag hollow alloy show the remarkably improved ORR kinetics, compared with the commercial Pt/C catalyst.For better understanding of the origin of the improved ORR activity, the specific activities (i.e., kinetic current per unit surface area of catalyst) for three Pt–Ag bimetallic catalysts were determined by normalizing the kinetic current against electrochemically surface area (ECSA) of each catalyst. The specific ECSA of these Pt-based catalysts can be estimated from cyclic voltammetry (CV) curves in N2-purged 0.5 M H2SO4 solution shown in Fig. 5d, by using the surface charge collected in the Hupd adsorption/desorption region, which has been reported as 210 μC cm−2 for the pure platinum.33 The obtained specific ECSA of these Pt-based catalysts are shown in Fig. 5e, while the corresponding specific activities of them are shown in Fig. 5f. As observed, the recorded specific activities, follow the order of Ag@Pt core–shell > hollow alloy > solid alloy > Pt/C. We noticed that the core–shell catalyst exhibits the specific activity of 0.12 mA cm−2, which is more than 7.6 times than that of the Pt/C catalysts (0.016 mA cm−2), demonstrating the improved ORR kinetics on the catalyst, anyway; the Pt–Ag hollow and solid alloy also display much higher specific activities than the commercial Pt/C. Therefore, we concluded that the high ORR activity of the Pt–Ag bimetallic catalysts can be achieved through the optimization of their nanophase structures. According to previous results reported in literature,11 the improved trend in ORR activity of Pt-based catalysts are usually attributed to the changes in their surface structures including the geometric (ensemble) and electronic structures, which can affect the ORR kinetics. In our case, the nanostructure-sensitive ORR activity for these Pt–Ag bimetallic catalysts most likely results from the modifications of the electronic structures within the synthesized bimetallic materials, which changes the interaction energy between oxygen and the catalysts, that is, the rate controlling step for ORR, and results in the improved ORR kinetics.34,35
3.4 The origin of improved activity of Pt–Ag bimetallic catalysts
To reveal the relationship between the electronic states in these Pt–Ag bimetallic catalysts with different nanostructures and their ORR activity, we performed the XPS measurements for them. The commercial Pt/C catalyst (E-TEK) and homemade Ag/C can be taken as reference materials. The resulting Pt 4f and Ag 3d core-level binding energies are shown in Fig. 6, respectively. It is obvious that all the Pt–Ag bimetallic catalysts exhibit the negative shift of Ag 3d binding energy and the positive shift of Pt 4f binding energy, relative to those in pure Ag/C and Pt/C samples respectively. It strongly implies the strong electronic interaction between Pt and Ag components in their bimetallic catalysts. To further explore the nature of the enhanced activities for these Pt-based catalysts as a result of the electronic interaction, we further fitted the XPS spectra in Fig. 7b by using XPSPEAK41 software with a Gaussian–Lorentzian line shape to determine various states of Pt 4f in these catalysts featuring core–shell, solid and hollow alloy nanostructures. As illustrated in Fig. 7a–c, the Pt 4f for the tested samples with different nanostructures contains various states, such as Pt(0) (metallic Pt) and Pt(II) species. Considering that the substantial shifts of the obtained binding energy of Pt(0) or Pt(II) represent the electronic interaction between Pt and Ag atoms, we compared the Pt(0) binding energies of these catalysts against their nanophase structures, as shown in Fig. 7d. It can be seen that the Pt(0) binding energy in Ag@Pt core–shell, hollow, and solid alloy NPs is shifted positively, relative to that of the commercial Pt/C (70.7 eV). The change in Pt(0) binding energy is at the following order: the hollow (0.2 eV) > the core–shell (0.09 eV) > the solid (0.03 eV) > Pt/C, revealing the correlation of the Pt(0) binding energy against their phase structures. The trend in the binding energy of Pt(0) for these bimetallic catalysts is in good agreement with the correlation of their mass-specific activities, indicating the significant impact of the core-level binding energy of Pt on the ORR kinetics. Moreover, a similar tendency of Pt(II) state is found in these catalysts against their nanophase structures and matched the ORR kinetics well. Therefore, we concluded that the enhanced ORR activity on these bimetallic catalysts may result significantly from the electronic interaction of two metals, which involved the electron transfer from Pt 4f to Ag 3d bands. Such unique electron transfer has been verified by the remarkable the positive shift of Pt 4f binding energy and negative shift of Ag 3d binding energy, as depicted from Fig. 6.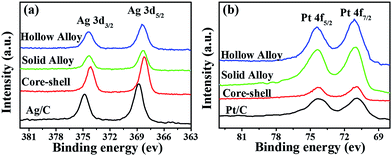 | ||
| Fig. 6 XPS measurements on the synthesized Ag@Pt core–shell NPs, solid and hollow Pt–Ag alloy NPs, and a commercial Pt/C (40 wt%, E-TEK). (a) Ag 3d spectra; (b) Pt 4f spectra. | ||
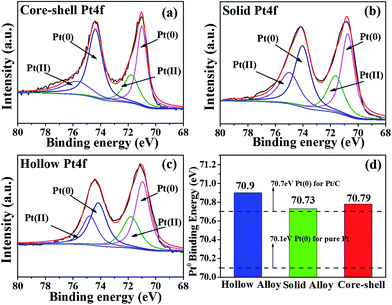 | ||
| Fig. 7 Fitting of Pt 4f XPS spectra of (a) the core–shell NPs, (b) solid alloy and (c) hollow alloy NPs; (d) Pt(0) binding energies of the above catalysts against their nanophase structures. | ||
Exact understanding of the impact of the electronic interaction between two metals on ORR activity is of interest and also particular important, because achieving synergy between Pt and other cheap metals is still a key challenge for the developing of highly active, economical bimetallic catalysts for ORR.36,37 In our case, the synergistic effect may originate from the substantial electron transfer from Pt 4f to Ag 3d in the Pt–Ag bimetallic catalysts, which is closely related to the enhanced activity for the ORR. There are several possibilities resulted in favourable ORR for these catalysts. On the one hand, the obviously increased binding energy of Pt 4f core level reflects the downshift of its d-band center relative to Fermi level, as a result of the electron donation from Pt to Ag atoms that makes the Pt atoms are subjected to a tensile force that narrows the d-orbital to generate the downshift.13,24 The downshift of the d-band center for Pt atoms can weakens the strongly chemical adsorption of oxygenate intermediates like OH− and thereby facilitates more favorable O2 adsorption on the Pt surface, that is, the first step of the ORR,12 which contributes to enhance ORR activity, relative to that of pure Pt. This outcome is verified by the similar results on Pt–M alloys (M = Co, Ni, or Fe) suggested by many investigators.38,39 It has been evidenced that metals with higher d-band centers bind oxygen strongly, and the O–O bonds can be broken easily, as is the cases for Pt, Ru, Rh, and Pd. Conversely, metals with a lower d-band center bind oxygen less strongly and thus the O–O bond is not split as efficiently, such as the coin metals, Au, Ag and Cu.1
On the other hand, the electron transfer can alter the electronic structure around Ag atoms in these Pt–Ag bimetallic catalysts, as evidenced by the negative shift in Ag 3d binding energy, caused by the strongly electronic interaction between two metals shown in Fig. 6 and 7. Interestingly, the strong electron transfer would lead to the increase in the numbers of the silver's unpaired electrons in the Pt–Ag alloy catalysts, according to the well-known Pauli exclusion principles. It is believed that active oxygen can preferential adsorbs on the metals with the number of unpaired electrons, because each oxygen atom requires two unpaired electrons from the d-band for bond formation.13 Although the influence of electronic states of Ag on the ORR processes is still debated, it is evident that pure silver like Cu and Au, having no any unpaired electrons and any d-band vacancy (fully filled bonding and antibonding states), displays the low interaction energy between oxygen and Ag.14 Thus, the increase unpaired electrons for Ag atoms donated form adjacent Pt atoms contributes to the formation of the partially filled bonding and antibonding states of Ag atoms,13 which result in the favorable adsorption of active oxygen on Ag atom surface, which would further facilitate the ORR kinetics on these Pt–Ag bimetallic catalysts.
Therefore, for the Pt–Ag bimetallic system, the physical origin of the enhancement in the ORR activity may be associated with a synergy raised by the interatomic electron transfer between Pt and Ag components in their bimetallic catalysts. The interatomic electronic transfer may also be associated with so-called hybridization effect that involves an increased d to sp promotion of metals.40 Abrikosov et al. have explained this behaviour in terms of intra-atomic electronic re-distribution due to valence electron hybridization.41 In our cases, the higher ORR activities of Ag@Pt core–shell and hollow NPs is due to the higher hybridization effects due to their unique nanostructure in comparison to that of the synthesized solid Pt–Ag NPs. This finding confirms that the synergistic effect between two metals, raised by changes in their electron configurations, can efficiently enhance ORR activity of Pt–Ag bimetallic catalysts, compared to each of pure metal. The synergistic effects can be achieved by manipulating their nanophase structures that is responsible for the remarkably improved electrocatalytic activity for towards ORR on these Pt–Ag bimetallic catalysts; thus, this approach promises a much more flexible catalyst design.
4. Conclusions
In summary, we have demonstrated a simple and effective route for the preparation of carbon-supported Pt–Ag bimetallic nanoparticles with Agcore@Ptshell core–shell, highly alloyed solid and hollow structures, using Ag NPs as the sacrificial templates via a galvanic replacement reaction in an aqueous solution containing H2PtCl6. The control of nanophase structures for the Pt–Ag bimetallic particles is achieved by simply regulating concentrations of H2PtCl6 in an aqueous solution. These nanoparticles are deposited on the surface of carbon black with average size of 10–15 nm. The formation of these Pt–Ag NPs with different nanostructures is closely attributed to a defect-induced Kirkendall effect, which involves the outward diffusion of Ag atoms from the inside to the surface and the inward diffusion of deposited Pt atoms induced by the high density defects along the temple NP surface. The electrocatalysis experiments in an acidic solution reveal a strong correlation between ORR activity and the nanostructures of the catalysts. In this Pt–Ag system, the core–shell and hollow alloy NPs exhibit the excellent mass and specific activities toward the ORR, which is remarkably higher than those of the commercial Pt/C (E-TEK). The improvement of the ORR activity may originate from synergistic effect from the hybridization effects and/or intra-atomic electronic re-distribution between Pt and Ag components in their bimetallic systems, relative to each metal. So, achieving a synergy between Pt and Ag is essential for the development of highly active bimetallic Pt–Ag catalysts for the ORR. This work proposed a new method to design of active and robust Pt-based bimetallic nanoparticle catalysts with controllable nanoscale phase structures that dominate the catalytic properties.Acknowledgements
This work was supported by National Natural Science Funds of China (Grant No. 50972003, 51432003).References
- J. Liu, J. Liu, W. Song, F. Wang and Y. Song, J. Mater. Chem. A, 2014, 2, 17477–17488 RSC.
- Z. Chen, M. Waje, W. Li and Y. Yan, Angew. Chem., Int. Ed., 2007, 46, 4060–4063 CrossRef CAS PubMed.
- E. Guilminot, A. Corcella, M. Chatenet, F. Maillard, F. Charlot, G. Berthomé, C. Iojoiu, J.-Y. Sanchez, E. Rossinot and E. Claude, J. Electrochem. Soc., 2007, 154, B1106–B1114 CrossRef CAS.
- W. Bi and T. F. Fuller, J. Electrochem. Soc., 2008, 155, B215–B221 CrossRef CAS.
- Y. Zhao, J. Liu, Y. Zhao and F. Wang, Phys. Chem. Chem. Phys., 2014, 16, 19298–19306 RSC.
- N. Toshima and T. Yonezawa, New J. Chem., 1998, 22, 1179–1201 RSC.
- F. H. Lima, C. D. Sanches and E. A. Ticianelli, J. Electrochem. Soc., 2005, 152, A1466–A1473 CrossRef CAS.
- H. Liu, F. Ye, Q. Yao, H. Cao, J. Xie, J. Y. Lee and J. Yang, Sci. Rep., 2014, 4, 3969 CrossRef PubMed.
- R. C. Sekol, X. Li, P. Cohen, G. Doubek, M. Carmo and A. D. Taylor, Appl. Catal., B, 2013, 138, 285–293 CrossRef.
- J. Zhang, M. B. Vukmirovic, Y. Xu, M. Mavrikakis and R. R. Adzic, Angew. Chem., Int. Ed., 2005, 44, 2132–2135 CrossRef CAS PubMed.
- V. R. Stamenkovic, B. S. Mun, M. Arenz, K. J. Mayrhofer, C. A. Lucas, G. Wang, P. N. Ross and N. M. Markovic, Nat. Mater., 2007, 6, 241–247 CrossRef CAS PubMed.
- Y.-Y. Feng, G.-R. Zhang, J.-H. Ma, G. Liu and B.-Q. Xu, Phys. Chem. Chem. Phys., 2011, 13, 3863–3872 RSC.
- F. Lima, J. Zhang, M. Shao, K. Sasaki, M. Vukmirovic, E. Ticianelli and R. Adzic, J. Phys. Chem. C, 2007, 111, 404–410 CrossRef CAS.
- A. Dhouib and H. Guesmi, Chem. Phys. Lett., 2012, 521, 98–103 CrossRef CAS.
- D. A. Slanac, W. G. Hardin, K. P. Johnston and K. J. Stevenson, J. Am. Chem. Soc., 2012, 134, 9812–9819 CrossRef CAS PubMed.
- S.-M. Jeong, M. K. Kim, G.-P. Kim, T. Y. Kim and S.-H. Baeck, Chem. Eng. J., 2012, 198, 435–439 CrossRef.
- D. Mott, J. Luo, P. N. Njoki, Y. Lin, L. Wang and C.-J. Zhong, Catal. Today, 2007, 122, 378–385 CrossRef CAS.
- S. Song, X. Wang, S. Li, Z. Wang, Q. Zhu and H. Zhang, Chem. Sci., 2015, 6, 6420–6424 RSC.
- S. Song, R. Liu, Y. Zhang, J. Feng, D. Liu, Y. Xing, F. Zhao and H. Zhang, Chem.–Eur. J., 2010, 16, 6251–6256 CrossRef CAS PubMed.
- X. Wang, Y. Zhang, S. Song, X. Yang, Z. Wang, R. Jin and H. Zhang, Angew. Chem., Int. Ed., 2016, 55, 4542–4546 CrossRef CAS PubMed.
- C. M. Doudna, M. F. Bertino, F. D. Blum, A. T. Tokuhiro, D. Lahiri-Dey, S. Chattopadhyay and J. Terry, J. Phys. Chem. B, 2003, 107, 2966–2970 CrossRef CAS.
- B. N. Wanjala, J. Luo, R. Loukrakpam, B. Fang, D. Mott, P. N. Njoki, M. Engelhard, H. R. Naslund, J. K. Wu and L. Wang, Chem. Mater., 2010, 22, 4282–4294 CrossRef CAS.
- J. Luo, L. Wang, D. Mott, P. N. Njoki, Y. Lin, T. He, Z. Xu, B. N. Wanjana, I. Lim and S. Im, Adv. Mater., 2008, 20, 4342–4347 CrossRef CAS.
- D. Zhao, Y.-H. Wang, B. Yan and B.-Q. Xu, J. Phys. Chem. C, 2009, 113, 1242–1250 CrossRef CAS.
- Y. Sun, B. Mayers and Y. Xia, Adv. Mater., 2003, 15, 641–646 CrossRef CAS.
- S. Yu, Q. Lou, K. Han, Z. Wang and H. Zhu, Int. J. Hydrogen Energy, 2012, 37, 13365–13370 CrossRef CAS.
- H. J. Fan, U. Gösele and M. Zacharias, Small, 2007, 3, 1660–1671 CrossRef CAS PubMed.
- E. González, J. Arbiol and V. F. Puntes, Science, 2011, 334, 1377–1380 CrossRef PubMed.
- T. Shahrabi, R. Newman and K. Sieradzki, J. Electrochem. Soc., 1993, 140, 348–352 CrossRef CAS.
- T. Shibata, B. A. Bunker, Z. Zhang, D. Meisel, C. F. Vardeman and J. D. Gezelter, J. Am. Chem. Soc., 2002, 124, 11989–11996 CrossRef CAS PubMed.
- W. Wang, M. Dahl and Y. Yin, Chem. Mater., 2012, 25, 1179–1189 CrossRef.
- W. Wei, Z. Wang, Z. Liu, Y. Liu, L. He, D. Chen, A. Umar, L. Guo and J. Li, J. Power Sources, 2013, 238, 376–387 CrossRef CAS.
- K. A. Kuttiyiel, K. Sasaki, Y. Choi, D. Su, P. Liu and R. R. Adzic, Nano Lett., 2012, 12, 6266–6271 CrossRef CAS PubMed.
- C.-L. Lee, Y.-J. Chao, C.-H. Chen, H.-P. Chiou and C.-C. Syu, Int. J. Hydrogen Energy, 2011, 36, 15045–15051 CrossRef CAS.
- F. Yuanyuan, M. Junhong, G. Zhang, Z. Dan and X. Boqing, Chin. J. Catal., 2009, 30, 776–779 CrossRef.
- B. Hammer and J. K. Nørskov, Adv. Catal., 2000, 45, 71–129 CAS.
- B. Hammer, Y. Morikawa and J. K. Nørskov, Phys. Rev. Lett., 1996, 76, 2141 CrossRef CAS PubMed.
- V. Stamenkovic, T. Schmidt, P. Ross and N. Markovic, J. Phys. Chem. B, 2002, 106, 11970–11979 CrossRef CAS.
- U. Paulus, A. Wokaun, G. Scherer, T. Schmidt, V. Stamenkovic, V. Radmilovic, N. Markovic and P. Ross, J. Phys. Chem. B, 2002, 106, 4181–4191 CrossRef CAS.
- S. K. Sengar and B. Mehta, J. Appl. Phys., 2014, 115, 124301 CrossRef.
- I. Abrikosov, W. Olovsson and B. Johansson, Phys. Rev. Lett., 2001, 87, 176403 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |