Stereoisomers of an azine-linked donor–acceptor conjugated polymer: the impact of molecular conformation on electrical performance†
Abstract
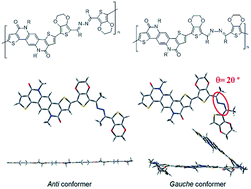
We herein report the synthesis of a pair of azine linked donor–acceptor type conjugated polymers by the use of palladium(II)-based direct arylation. The two stereoisomers of the azine molecule were synthesized, separated, characterized, and further incorporated with a thiophene–phenylene–thiophene-based fused lactam (TPTL) acceptor molecule to form the donor–acceptor skeletons. The effects of the azine linkage isomerism on the polymer skeleton were studied both theoretically and experimentally. Semiconducting properties of these polymers were also evaluated in thin film transistors.

 Please wait while we load your content...
Please wait while we load your content...