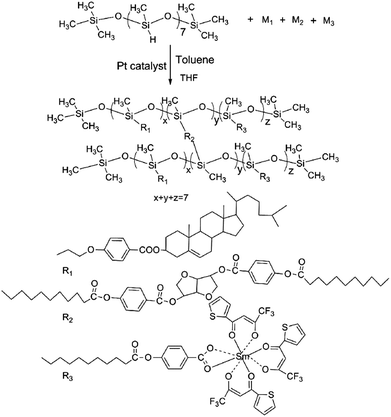
Novel luminescent chiral network liquid-crystalline polymers containing Sm(III) ions†
Bing Yao,
Yuehua Cong,
Baoyan Zhang*,
Zeqing Wang,
Fei Chen and
Cong Sun
Research Centre for Molecular Science and Engineering, Northeastern University, Wenhua Road, Heping District, Shenyang, 110004, PR China. E-mail: byzhang2005@126.com
First published on 21st April 2016
Abstract
Novel luminescent chiral network liquid-crystalline polymers containing Sm(III) ions (Sm-LCPs) exhibiting wonderful liquid crystalline properties and unique fluorescence properties were prepared by poly(methylhydrogeno)siloxane (PMHS), chiral liquid crystal monomer M1, crosslinking agent M2 and samarium complex M3. The chemical structures, liquid-crystalline behaviours of Sm-LCPs were characterized by various experimental techniques. The introduction of small amount of samarium ions endowed liquid-crystalline polymers with fascinating fluorescence properties. All Sm-LCPs were chiral, and samarium ions did not marked affect the textures of liquid-crystalline polymers. Fourier transform infrared imaging study exhibited that samarium ions were evenly distributed in polymers. A spiral structural model was established to demonstrate the distribution and interaction of components. All Sm-LCPs showed wide mesophase temperature ranges, reversible mesomorphic phase transitions and high thermal stability. The thermogravimetric analysis (TGA) results displayed that the decomposition temperatures (5% weight loss) of all Sm-LCPs were greater than 297 °C. The Sm-LCPs can emit soft red light when excited. Fluorescence intensity of Sm-LCPs gradually increased with increase in samarium complexes from 0.2 to 1.0 mol%. The temperature dependence of luminescence intensity was studied, where the fluorescence intensity of Sm-LCPs decreased monotonically with increase in temperature.
1. Introduction
Lanthanide luminescent complexes have made an immense contribution to the rapid development of functional materials. They exhibit excellent property of emitting particularly efficient strong narrow-width spectra in a visible light region,1–3 and they possess potential applications in biological investigations,4 light emission molecular devices,5 lasers,6 drug delivery and sensors,7–9 etc. However, the low thermal stability and high phase inversion temperature are major defects which hamper their practical applications.10,11 Encouraged by overcoming these shortcomings and superior fluorescence materials, some attempts have been made to solve these problems by combining lanthanide complexes with inert matrices – for instance, sol–gel glasses,12–14 liquid crystal (LC) materials,15–17 polymers,18,19 or organic–inorganic hybrid materials.20,21In addition to the above-mentioned methods, chiral network liquid crystalline polymers seem to be promising candidates due to their excellent thermal performance, processability, unique optical properties such as thermochromism, selective reflection of light, circular dichroism,22–26 and their potential applications in full-color liquid crystal displays, nonlinear optical materials, switchable storage devices, lasers, anisotropic light emitters,27–31 etc. The introduction of fluorescent samarium complexes into the ordered spiral matrices of chiral network LCPs is beneficial for both of them. First, chiral network LCPs endow fluorescent samarium complexes with excellent thermal performance and processability. Second, the ordered spiral structures of chiral network LCPs are conductive to uniform distribution of samarium complexes, which improve the luminous efficiency of samarium ions. Last but not the least, samarium complexes confer self-luminescence property on the chiral network liquid crystalline polymers. It is promising that luminescent chiral network LCPs materials lead to new technical applications in many fields such as mechanics, electronics, optics, displays, etc. As a consequence, developing multifarious luminescent chiral network LCPs is of great practical significance.
In this paper, a series of novel chiral network Sm-LCPs were synthesized by graft copolymerization. A spiral schematic illustration of Sm-LCPs was established to demonstrate the interaction and distribution of components. Sm-LCPs can emit soft red light when excited. Luminescence intensity of Sm-LCPs gradually increased with increase in lanthanide complexes but decreased monotonically with increase in temperature.
2. Experimental
2.1. Materials
Poly(methylhydrogeno)siloxane (PMHS, Mn = 582) are obtained from Jilin Chemical Industry Co. (Jilin City, China); 3-bromopropene, 4-hydroxybenzoic acid, undecylenic acid, isosorbide, sulfurous dichloride, isopropanol, sodium isopropoxide, toluene, benzene, chloroform, ethyl alcohol, tetrahydrofuran (THF) and pyridine were purchased from Shenyang Chemical Co. (China); cholesterol was purchased from Henan Xiayi Medical (China); anhydrous SmCl3 and 2-thenoyltrifluoroacetone (TTA) was purchased from Sinopharm Chemical Reagent Co. (China); toluene and tetrahydrofuran were purified by distillation over sodium hydride before use. All other solvents and reagents were purified by standard methods.2.2. Measurements
Fourier transform infrared (FTIR) spectra of the synthesised samples were obtained using the KBr method performed on a PerkinElmer Instruments Spectrum One Spectrometer (PerkinElmer, Foster City, CA, USA). 1H NMR (600 MHz) spectra were measured using a Varian WH-90PFT NMR Spectrometer (Varian Associates, Palo Alto, CA). Elemental analyses (EA) were carried out using an Elementar Vario ELIII (Elementar, Germany). The polarised optical microscopy (POM) study was performed with a Leica DMRX (Leica, 90Wetzlar, Germany) equipped with a Linkam THMSE-600 (Linkam, Surrey, UK) heating stage. Fourier transform infrared imaging was performed using a Spotlight 300 infrared imaging system (PerkinElmer). Specific rotations of samples were measured by WZZ-2S Automatic Polarimeter. The thermal stability of Tb-LCI was measured under a nitrogen atmosphere with a Netzsch TGA 209C thermogravimetric analyser at a heating rate of 10 °C min−1. A HORIBA Jobin Yvon FL3-TCSPC fluorescence spectrophotometer was used for fluorescence measurements. Thermal transition properties were characterised using a Netzsch Instruments DSC 204 (Netzsch, Wittelsbacherstr, Germany) at heating and cooling rates of 10 °C min−1 under a nitrogen atmosphere.2.3. Synthesis of olefinic monomers
The synthesis of M1, M2, M3 and intermediate UBA were shown in Fig. 1. M1 was synthesized via a previously published method,32 UBA and M2 were synthesized via a previously published method.33 M1 and M2 were explained in ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was heated to 50 °C for 12 h under N2 and then added a solution of sodium isopropoxide (2.46 g, 30.0 mmol) in 20 mL of isopropanol. The mixture was refluxed for 4 h to synthesize samarium isopropoxide and a solution of 2-thenoyltrifluoroacetone (6.66 g, 30.0 mmol) dissolved in 30 mL of benzene was added dropwise to the stirred solution. After the solution was refluxed for 2.5 h, a solution of UBA (3.04 g, 10.0 mmol) in 10 mL of THF was added. The reactive mixture was refluxed for 3 h. After cooling to room temperature, the mixture was filtered. The products were obtained after the solvent evaporated and washed three times with cyclohexane and dried at 30 °C in a vacuum for 12 h. Yield: 64%. IR (KBr): 3091 (
1) was heated to 50 °C for 12 h under N2 and then added a solution of sodium isopropoxide (2.46 g, 30.0 mmol) in 20 mL of isopropanol. The mixture was refluxed for 4 h to synthesize samarium isopropoxide and a solution of 2-thenoyltrifluoroacetone (6.66 g, 30.0 mmol) dissolved in 30 mL of benzene was added dropwise to the stirred solution. After the solution was refluxed for 2.5 h, a solution of UBA (3.04 g, 10.0 mmol) in 10 mL of THF was added. The reactive mixture was refluxed for 3 h. After cooling to room temperature, the mixture was filtered. The products were obtained after the solvent evaporated and washed three times with cyclohexane and dried at 30 °C in a vacuum for 12 h. Yield: 64%. IR (KBr): 3091 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2928–2853 (–CH2–), 1714 (C
CH), 2928–2853 (–CH2–), 1714 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1603, 1572 (Ar–), 1257 (C–O), 1167 (C–F). Anal. calcd for C42H35F9O10S3Sm: C 45.15, H 3.16, O 14.32; found: C 45.02, H 3.15, O 14.34.
O), 1603, 1572 (Ar–), 1257 (C–O), 1167 (C–F). Anal. calcd for C42H35F9O10S3Sm: C 45.15, H 3.16, O 14.32; found: C 45.02, H 3.15, O 14.34.2.4. Synthesis of luminescent chiral network LCPs
All polymers were obtained by the same synthesis methods as shown in Fig. 2. Polymerization experiments were summarized in Table 1. The synthesis of Sm-P1 is presented as an example. Liquid crystal monomer M1 (0.4910 g, 0.898 mmol) was dissolved in 30 mL of dry, freshly distilled toluene, monomer M2 (0.0719 g, 0.100 mmol), M3 (0.0022 g, 0.002 mmol) and poly(methylhydrogeno)siloxane (PMHS, 0.0831 g, 0.1428 mmol) dissolved in 30 mL THF were added to the stirred solution, 0.2 mL of a 0.5% hexachloroplatinic THF solution was added to the stirred solution and heated under nitrogen and anhydrous condition at 90 °C for 48 h. The reaction was monitored by following the disappearance of Si–H band at 2166 cm−1 in FTIR spectra. Complete disappearance of Si–H band indicated successful incorporation of monomers into polysiloxane chains. After cooling to room temperature, the solution was poured into 200 mL methanol, after filtration, the products were washed with anhydrous hot ethyl alcohol (three times) and dried at 45 °C in a vacuum oven for 12 h to obtain polymer Sm-P1. Yield: 82%. IR (KBr): 2927–2855 (–CH3, –CH2–), 1742–1727 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1605, 1510 (Ar–), 1127–1007 (Si–O–Si).
O), 1605, 1510 (Ar–), 1127–1007 (Si–O–Si).
| Sample | Feed (mmol) | Sm3+ (mol%) | Specific rotationsa | |||
|---|---|---|---|---|---|---|
| PMHS | M1 | M2 | M3 | |||
| a Specific rotations were measured when polymers were dissolved in THF (ρ = 0.1 g/100 mL). | ||||||
| P0 | 0.1428 | 0.900 | 0.100 | 0.000 | 0 | −8.97 |
| Sm-P1 | 0.1428 | 0.898 | 0.100 | 0.002 | 0.2 | −8.91 |
| Sm-P2 | 0.1428 | 0.896 | 0.100 | 0.004 | 0.4 | −8.86 |
| Sm-P3 | 0.1428 | 0.894 | 0.100 | 0.066 | 0.6 | −8.74 |
| Sm-P4 | 0.1428 | 0.892 | 0.100 | 0.008 | 0.8 | −8.69 |
| Sm-P5 | 0.1428 | 0.890 | 0.100 | 0.010 | 1.0 | −8.65 |
3. Results and discussion
3.1. Optical textures
Fig. 3 displays some representative optical textures which were obtained by POM with hot stage on the first heating cycles.Optical textures of liquid crystal monomer M1 were studied. POM results showed that M1 exhibited enantiotropic oily streak texture and focal conic texture of cholesteric phase during the heating and cooling cycles. The oily streak texture of M1 is shown in Fig. 3(a). Fig. 3(b)–(e) illustrates some representative optical textures of P0, Sm-P1, Sm-P3 and Sm-P5, all polymers showed similar cholesteric phase textures indicating that samarium complexes did not marked affect the liquid crystalline textures of polymers. Take sample Sm-P1 as an example, when it was heated above 46.7 °C, eyesight became bright and cholesteric Grandjean texture gradually appeared. With the temperature rising, Grandjean texture became bright in color as shown in Fig. 3(b) and disappeared at 182.2 °C. Similarly, when the melt was cool, the cholesteric Grandjean texture appeared again.
Opticity is one of the important optical properties of chiral liquid crystalline materials. It is the proof of chiral liquid crystalline materials. The opticity study showed that all polymers were chiral in accordance with the POM results. The specific rotations are summarized in Table 1.
3.2. Distribution of samarium ions in Sm-LCPs
Carboxylates exhibit characteristic absorptions at 1419 cm−1. Subsequently, the total infrared absorptions of Sm-LCPs were tested by Fourier transform infrared imaging system to analyse the distribution of samarium ions in polymers. The samples were dissolved in anhydrous isopropanol and chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to prepare the same concentration of solution. Same volume of solution was put onto KBr window, test the sample after the solvent evaporation. Finally the characteristic infrared absorptions of carboxylates at 1419 cm−1 were marked in the total absorptions of Sm-LCPs, the infrared imaging of Sm-P2 and Sm-P5 are displayed in Fig. 4. Green areas represent weak absorptions at 1419 cm−1, yellow and red areas represent strong absorptions at 1419 cm−1. It is obvious that the samarium ions were evenly distributed in polymers instead of forming clusters, furthermore, the content of samarium ions in Sm-LCPs was in the order of Sm-P5 > Sm-P4 > Sm-P3 > Sm-P2 > Sm-P1, – consistent with our theory.
5) to prepare the same concentration of solution. Same volume of solution was put onto KBr window, test the sample after the solvent evaporation. Finally the characteristic infrared absorptions of carboxylates at 1419 cm−1 were marked in the total absorptions of Sm-LCPs, the infrared imaging of Sm-P2 and Sm-P5 are displayed in Fig. 4. Green areas represent weak absorptions at 1419 cm−1, yellow and red areas represent strong absorptions at 1419 cm−1. It is obvious that the samarium ions were evenly distributed in polymers instead of forming clusters, furthermore, the content of samarium ions in Sm-LCPs was in the order of Sm-P5 > Sm-P4 > Sm-P3 > Sm-P2 > Sm-P1, – consistent with our theory.
Combining the analysis results of POM, opticity and Fourier Transform Infrared Imaging, a spiral structural model of Sm-LCPs was established to demonstrate the interaction and distribution of components as shown in Fig. 5. Samarium complexes are evenly distributed in spiral matrices of LCPs. Sm-LCPs can emit soft red light when excited.
3.3. Thermal properties
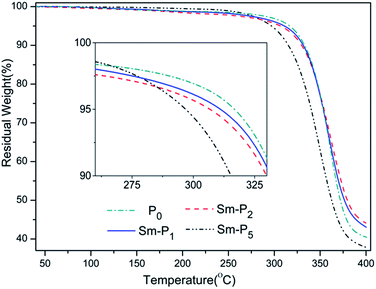
For P0 and Sm-LCPs, the quantised values of glass transition temperatures (Tg) and mesophase–isotropic phase transition temperatures (Ti) were obtained from the dedicated software of the Netzsch instrument. Table 2 shows decomposition temperatures (Td) and phase transition temperatures. DSC curves are illustrated in Fig. 6(a), the effects of samarium complexes on phase transition temperatures are shown in Fig. 6(b). All Sm-LCPs displayed obvious glass transitions and mesophase–isotropic phase transitions, suggesting that samarium complexes did not change the liquid crystalline state. As seen from Table 2 and Fig. 6, glass transition temperature (Tg) values of Sm-LCPs decreased with increase in samarium complexes ranging from 47.4 to 43.3 °C due to the decrease of rigid mesogens and increase of flexible segments. Ti value of Sm-LCPs decreased with an increase concentration of samarium complex units (0.2–1.0 mol%), the reason for the decreased Ti is that the samarium complex units affected the regularity of chain segments and the mobility, orientation of chiral liquid crystalline mesogenic units. All Sm-LCPs displayed wide mesophase temperature ranges (ΔT) as shown in Table 2.| Sample | DSC | ΔTa | TG | |
|---|---|---|---|---|
| Tg (°C) | Ti (°C) | T5%b (°C) | ||
| a Mesophase temperature ranges (Ti–Tg).b Temperature at which 5% weight loss occurred. | ||||
| P0 | 47.4 | 183.7 | 136.3 | 314.5 |
| Sm-P1 | 46.7 | 182.2 | 135.5 | 310.5 |
| Sm-P2 | 45.9 | 180.8 | 134.9 | 305.1 |
| Sm-P3 | 44.8 | 179.6 | 134.8 | 308.3 |
| Sm-P4 | 44.1 | 177.5 | 133.4 | 303.7 |
| Sm-P5 | 43.3 | 175.3 | 132.0 | 297.6 |
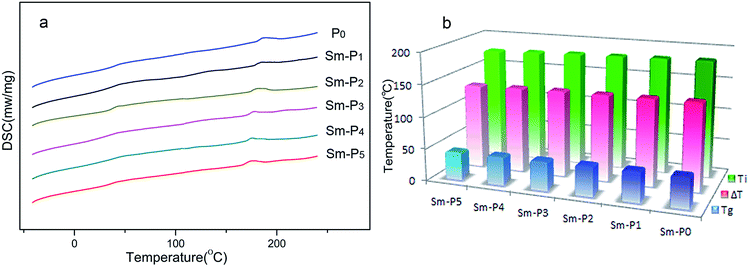 | ||
| Fig. 6 (a) DSC thermograms of P0 and Sm-LCPs. (b) The effects of samarium complexes on phase transition temperatures. | ||
The thermal stability of polymers which is related to molecular structure and molecular weight is important to estimate their working temperature limits and environmental conditions. Temperature for 5 wt% loss is always used as Td to estimate thermal stability of a polymer. Some representative TGA curves are illustrated in Fig. 7. Td of all Sm-LCPs took place above 297 °C showing good thermal stability, which indicated that the stable silicone backbones of chiral network LCPs prominently strengthened the thermal stability of samarium complexes and broadened the working temperatures.
3.4. Luminescence properties of Sm-LCPs
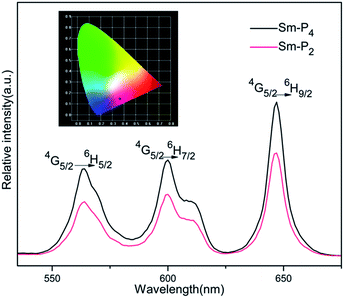
Sm-LCPs in LC phases were obtained by rapid freezing in a liquid nitrogen-vitrified mesophase. Luminescence properties of Sm-LCPs in LC states were examined. The typical emission spectra of Sm-P2 and Sm-P4 are presented in Fig. 8. Excitation spectrum wavelength of M3 and Sm-LCPs was 397 nm, the emission lines near 564 nm, 600 nm and 647 nm were assigned to the 4G5/2 → 6HJ/2 (J = 5, 7, 9) transitions. The black dot on the color coordinates in Fig. 8 represents characteristic narrow-width soft red emission. For all samples, fluorescence intensity gradually increased with increase in samarium complexes. When Sm3+ (mol%) = 1.0%, Sm-LCPs did not show fluorescence quenching, due to samarium complexes being evenly distributed in the ordered spiral structure of chiral network liquid crystalline polymers, which increased the luminous efficiency of samarium ions.The temperature dependence of fluorescence intensity was measured to investigate the thermal stability of photoluminescence in the LC phases. Fig. 9 shows the emission spectra of Sm-P3 at different temperatures. The fluorescence intensity of Sm-P3 decreased monotonically with increase in temperature in the scope of study. The possible reason may be that, with the temperature increased, the molecular liquidity increased resulting in higher fluorescence quenching efficiency in the liquid crystalline phase when excited. Consistent with our theory, fluorescence intensity as a function of temperature fits well the well-known thermal activation function:34
I(T) = I0/[1 + α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−EA/KBT)] exp(−EA/KBT)] |
4. Conclusions
In this paper, a series of novel luminescent chiral network liquid crystalline polymers containing samarium ions were synthesized and characterized. All Sm-LCPs are chiral. They displayed similar cholesteric Grandjean textures, which indicated that the introduction of small amount of samarium complexes did not change the liquid crystalline state of polymers. A spiral structural model was established to demonstrate that samarium complexes were uniformly distributed in the spiral matrices of chiral network LCPs. The fluorescent chiral network LCPs showed wide mesophase temperature ranges and obviously good thermal stability. All chiral network Sm-LCPs can emit soft red light when excited. Fluorescence intensity of chiral network Sm-LCPs gradually increased with increase in samarium complexes from 0.2 to 1.0 mol%. The ordered spiral structure of chiral network liquid crystalline polymers improved the uniform distribution of samarium complexes, which increased the luminous efficiency. Fluorescence intensity of chiral network Sm-LCPs decreased with increase in temperature in the liquid crystalline phase. It is feasible that the fluorescence intensity of chiral network Sm-LCPs can be quantitatively adjusted by accurately controlling the temperature. The combination of chiral liquid crystalline properties and fluorescence properties allows the potential to fabricate multi-functional optical materials.Acknowledgements
The authors are grateful to the National Key Technology Support Program of China (contract grant number 2008BAL55B03) and the Science and Technology Department of Liaoning Province for financial support of this work.References
- F. Y. Zheng, J. Zhang and S. Y. Feng, RSC Adv., 2013, 3, 9957–9964 RSC.
- L. Armelao, S. Quici, F. Barigelletti, G. Accorsi, G. Bottaro, M. Cavazzini and E. Tondello, Coord. Chem. Rev., 2010, 254, 487–505 CrossRef CAS.
- A. Ghatak, G. H. Debnath, M. Mandalb and P. Mukherjee, RSC Adv., 2015, 5, 32920–32932 RSC.
- A. K. Singh, S. K. Singhc and S. B. Raia, RSC Adv., 2014, 4, 27039–27061 RSC.
- Y. Ma and Y. Wang, Coord. Chem. Rev., 2010, 254, 972–990 CrossRef CAS.
- L. N. Sun, Y. N. Qiu, T. Liu, H. S. Peng, W. Deng, Z. J. Wang and L. Y. Shi, RSC Adv., 2013, 3, 26367–26375 RSC.
- W. Ahmad, L. J. Zhang and Y. S. Zhou, CrystEngComm, 2014, 16, 3521–3531 RSC.
- J. Garcia-Torres, P. Bosch-Jimenez, E. Torralba-Calleja, M. Kennedy, H. Ahmed, J. Doran, D. Gutierrez-Tauste, L. Bautista and M. Della Pirriera, J. Photochem. Photobiol., A, 2014, 283, 8–16 CrossRef CAS.
- K. Singh, S. Banerjeeb and A. K. Patra, RSC Adv., 2015, 5, 107503–107513 RSC.
- K. Binnemans, J. Mater. Chem., 2009, 19, 448–453 RSC.
- K. Binnemans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- L. N. Sun, Y. N. Qiu, T. Liu, H. S. Peng, W. Deng, Z. J. Wang and L. Y. Shi, RSC Adv., 2013, 3, 26367–26375 RSC.
- A. M. Klonkowski, S. Lis, M. Pietraszkiewicz, Z. Hnatejko, K. Czarnobaj and M. Elbanowski, Chem. Mater., 2003, 15, 656–663 CrossRef CAS.
- L. N. Sun, Y. N. Qiu, T. Liu, H. S. Peng, W. Deng, Z. J. Wang and L. Y. Shi, RSC Adv., 2013, 3, 26367–26375 RSC.
- Y. T. Yang, K. Driesen, P. Nockemann, K. Van Hecke, L. Van Meervelt and K. Binnemans, Chem. Mater., 2006, 18, 3698–3704 CrossRef CAS.
- T. Cardinaels, K. Driesen, T. N. Parac-Vogt, B. Heinrich, C. Bourgogne, D. Guillon, B. Donnio and K. Binnemans, Chem. Mater., 2005, 17, 6589–6598 CrossRef CAS.
- Y. G. Galyametdinov, A. A. Knyazev, V. I. Dzhabarov, T. Cardinaels, K. Driesen, C. Görller-Walrand and K. Binnemans, Adv. Mater., 2008, 20, 252–257 CrossRef CAS.
- L. H. Wang, W. Wang, W. G. Zhang, E. T. Kang and W. Huang, Chem. Mater., 2000, 12, 2212–2218 CrossRef CAS.
- K. Kuriki, Y. Koike and Y. Okamoto, Chem. Rev., 2002, 102, 2347–2356 CrossRef CAS PubMed.
- X. Lian and B. Yan, New J. Chem., 2015, 39, 5898–5901 RSC.
- Z. Y. Yan and B. Yan, New J. Chem., 2014, 38, 2604–2610 RSC.
- J. W. Wang and B. Y. Zhang, Liq. Cryst., 2013, 40, 1550–1560 CrossRef CAS.
- J. B. Guo, J. M. Zhang, Q. Zhang, N. Jiang and J. Wei, RSC Adv., 2013, 3, 21620–21627 RSC.
- S. J. Wezenberg, F. Ferroni, S. Pieraccini, W. B. Schweizer, A. Ferrarini, G. P. Spada and F. Diederich, RSC Adv., 2013, 3, 22845–22848 RSC.
- O. T. Picot, M. Dai, E. Billoti, D. J. Broer, T. Peijsab and C. W. M. Bastiaansenab, RSC Adv., 2013, 3, 18794–18798 RSC.
- P. C. Lin, Y. H. Cong and B. Y. Zhang, Appl. Mater. Interfaces, 2015, 7, 6724–6732 CrossRef CAS PubMed.
- M. O'Neill and S. M. Kelly, Adv. Mater., 2003, 15, 1135–1146 CrossRef.
- U. A. Hrozhyk, S. V. Serak, N. V. Tabiryan and T. J. Bunning, Adv. Funct. Mater., 2007, 17, 1735–1742 CrossRef CAS.
- H. H. Wang, L. Wang, M. Chen, T. D. Li, H. Cao, D. K. Yang, Z. Yang, H. Yang and S. Q. Zhu, RSC Adv., 2015, 5, 58959–58965 RSC.
- A. A. Khan, M. A. Bin-Kamarudin, P. R. Kidambi, S. Hofmann, T. D. Wilkinsona and M. M. Qasim, RSC Adv., 2015, 5, 57437–57443 RSC.
- G. Shanker and C. V. Yelamaggad, J. Phys. Chem. B, 2011, 115, 10849–10859 CrossRef CAS PubMed.
- J. S. Hu, B. Y. Zhang, Y. Guan and X. Z. He, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5262–5270 CrossRef CAS.
- B. Y. Zhang, J. S. Hu, B. Wang, D. S. Yao and H. Li, Colloid Polym. Sci., 2007, 285, 1683–1690 CAS.
- H. Zhang, H. W. Song, H. Q. Yu, X. Bai, S. W. Li, G. H. Pan, Q. L. Dai, T. Wang, W. L. Li, S. Z. Lu, X. G. Ren and H. F. Zhao, J. Phys. Chem. C, 2007, 111, 6524–6527 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fourier transform infrared (FTIR) spectra of the samples, 1H NMR (600 MHz) spectra of M1 and M2. See DOI: 10.1039/c6ra07388c |
| This journal is © The Royal Society of Chemistry 2016 |