Highly efficient and recyclable alkylammonium hydrosulfate catalyst for formation of bisphenol F by condensation of phenol with formaldehyde†
Jiafu Xiaoa,
Hua Huangab,
Weijian Xianga,
Wei Liaoa,
Junyi Liuab,
Xichun Sheb,
Qiong Xua,
Zaihui Fua,
Steven Robert Kirk a and
Dulin Yin*a
a and
Dulin Yin*a
aNational & Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources, College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, PR China. E-mail: dulinyin@126.com
bHunan Changling Petrochemical S&T Developing Co. Ltd, Yueyang 414012, PR China
First published on 22nd September 2016
Abstract
Several C1–C4 alkylammonium hydrosulfates [R3NH][HSO4] have been conveniently prepared from the cheap raw materials sulfuric acid and alkylamines. Their acidities were measured by chemical titration and determined using UV-vis spectroscopy with a basic indicator 4-nitroaniline. The catalytic performance of these hydrosulfates for the condensation of phenol with formaldehyde to bisphenol F (BPF) was evaluated in detail on a batch reactor. The results indicated that the proposed catalysts are very active for such condensation due to its homogeneous catalysis characteristics in reaction conditions. Among the catalysts examined, [H3NCH2CH2NH3][HSO4]2 shows the best catalytic performance and it can achieve a complete conversion of formaldehyde, providing a higher than 90% selectivity for BPF under the optimal conditions. Furthermore, the catalyst can be recovered from the reaction mixture via an azeotropic distillation with cyclohexane to remove water and then filtration and used repeatedly six times almost without loss of activity, showing an excellent reusability. It is suggested that the present catalytic process combines the characteristics of a homogenized reaction and heterogenized recovery so might provide a highly-efficient, environmentally-friendly and low-cost route for synthesis of bisphenol F.
1. Introduction
Bisphenol F (BPF) is a common name for a mixture of p,p′-dihydroxyphenolmethane, o,p′-dihydroxyphenolmethane and o,o′-dihydroxyphenolmethane, often used as a raw material for the synthesis of low viscosity epoxy resins, polyester and polycarbonate resins. It is also used as a modifier and stabilizer for phenolic resins, etc. In the last several decades,1 BPF has attracted considerable attention due to its attractive applications compared with bisphenol A. BPF is conventionally prepared by condensation of phenol with formaldehyde using some liquid mineral acids (phosphoric acid or sulfuric acid) as catalysts,2–5 which has several disadvantages of separation, recycling, treatment of spent acids, and equipment corrosion. In order to overcome the drawbacks of homogeneous catalysis, some recyclable solid acids have been employed to develop a heterogeneous catalysis process for the synthesis of bisphenol F, they include aluminum-grafted MCM-41 molecular sieves,6,7 H-beta zeolite,8 dodecatungstophosphoric acid (DTP) impregnated on fumed silica,9 montmorillonite K10 (ref. 10) or activated-bentonite,11 cesium-substituted DTP-based solid composite catalysts xCsTPA–ZTP12 and mesoporous M (Al, Zr, Al–Zr)-SBA-15.13 The solid acids14 used are, however, easily deactivated during reaction because polycondensates formed from the consecutive condensation of bisphenol F with formaldehyde were deposited inside their pores. As a result, a complicated regeneration process including reflux process with organic solvent, then an additional calcination or activated process to remove such sediments inside the catalyst's pores, is required to recover the activity of catalyst.Recently, some ionic liquid acids have attracted substantial attention due to their special physical and chemical properties.15–19 A thermoregulated phase-separable reaction system based on a series of water-soluble imidazolium-based Brønsted acids as catalysts was designed to achieve the highly efficient synthesis of bisphenol, which should be attribute to its advantage in combing homogenous catalytic reaction with heterogenous separation of catalyst.20 Only part of the catalyst used, however, can be separated in this system and separation of the residual water-soluble catalyst still needs to be extracted with water from reaction mixtures and further improved in efficiency.
In order to further decrease the cost of catalyst and improve its catalysis and recovery efficiencies, we attempt to establish a highly efficient catalysis system with the characteristics of homogenized reaction and heterogenized catalyst recovery for the condensation of phenol with formaldehyde to BPF. In that, a series of alkylammonium hydrosulfates prepared via cheap alkylamines and sulfuric acid as raw materials are used to homogeneously catalyze the condensation. After reaction, they can be recovered from the reaction mixture via the removal of water using cyclohexane's azeotropic distillation and then filtration. Herein, we would report some initial results obtained from employing this catalytic process for highly efficient synthesis of BPF.
2. Experimental
2.1 Chemicals and materials
The starting materials phenol, formaldehyde, trimethylamine, triethylamine, N-butylamine, N-tripropylamine, sulfuric acid, ethylenediamine, 1,2-propanediamine, phenolphthalein, sodium hydroxide, acetylacetone and 4-nitroaniline were AC grade, while potassium hydrogen phthalate was GC grade and purchased from Sinopharm chemical Reagent Co., Ltd. Bisphenol F (99%) standard was received from Alfa Aesar. All chemicals were used as received without any purification process.2.2 Synthesis and characterization of alkylammonium hydrosulfates
According to the reported preparation of the simple ammonium based acid catalysts,21,22 the synthesis of alkylammonium hydrosulfates was modified. A 100 mL round-bottomed glass reactor equipped with a reflux condenser and a thermometer was immersed in a recirculating heated oil-bath. 0.1 mol sulfuric acid was dropped slowly into the 0.1 mol alkylamine at 20 °C for 60 min. The mixture was then stirred for an additional 120 min at 60 °C to ensure the reaction finished completely. Finally, the resulting reaction solution was completely evaporated to dryness at 70 °C under vacuum (5 mm Hg) to yield the white solid product.The 1H NMR spectra of the synthesized alkylammonium hydrosulfates were recorded with a 500 MHz Bruker spectrometer in DMSO-d6 calibrated with tetramethylsilane (TMS) as the internal reference. Thermogravimetric analysis of the samples under nitrogen flow was conducted in a Netzsch-STA409PC thermal gravimetric analyzer. During the analysis, the temperature was increased from room temperature to 800 °C at a heating rate of 10 °C min−1. Melting point was determined by a WRS-1B digital melting point apparatus at a linear heating rate of 1 °C min−1.
The detailed 1H NMR data, melting point and thermal decomposition point of alkylammonium hydrosulfates are shown below:
2.3 Measurement of acidity of alkylammonium hydrosulfates
A traditional acid–base titration was used to determine the acidic amount of the alkylammonium hydrosulfates, 1 mmol alkylammonium hydrosulfates was dissolved in 20 mL distilled water, then titrated by the calibrated 0.05 mol L−1 NaOH solution and phenolphthalein as an indicator. The acid amount of catalyst could be obtained based on the consumption amount of NaOH solution.The Brønsted acidic strength of catalysts was evaluated by Hammett acidity functions and UV-vis spectroscopy with a basic indicator reported by Thomazeau.23 According to Thomazeau's method, the protonation extent of the basic indicator 4-nitroaniline (named I) in ionic water was determined. The molar concentration of the unprotonated indicator base may be represented by [I] and [IH+] is the molar concentrations of the protonated indicator base in a solution; pK(I)aq is the pKa value of the indicator base in aqueous solution (pK(I)aq = 0.99). The resulting Hammett function H0 was defined as
| H0 = pK(I)aq + log([I]/[IH+]) | (1) |
2.4 Catalyst evaluation
The condensation of phenol with formaldehyde catalyzed by different alkylammonium hydrosulfates catalysts were carried out in a 50 mL magnetically stirred round-bottomed flask equipped with a reflux condenser and a thermometer. In a typical condensation reaction, 100 mmol phenol, 10 mmol aqueous formaldehyde (37%) and 0.5 mmol catalyst were added into the reactor, which then was heated to 70 °C for 2 h. The products were determined with the external standard method using an Agilent 1100 HPLC equipped with a Venusil C18 (4.6 mm × 250 mm); a methanol–water (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35) mixture was used as mobile phase and solvent. The unreacted formaldehyde was determined using the Shimadzu UV-2450 spectrophotometer using an acetylacetone spectrophotometric method.24 The catalytic activities of these hydrosulfates were evaluated in terms of formaldehyde conversion and the selectivity of bisphenol F and its isomer distribution, which were calculated as below:
35) mixture was used as mobile phase and solvent. The unreacted formaldehyde was determined using the Shimadzu UV-2450 spectrophotometer using an acetylacetone spectrophotometric method.24 The catalytic activities of these hydrosulfates were evaluated in terms of formaldehyde conversion and the selectivity of bisphenol F and its isomer distribution, which were calculated as below:
 | (2) |
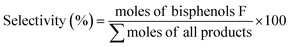 | (3) |
2.5 Catalyst reusability
When the condensation reaction proceeded until free formaldehyde was no longer detected, the reflux condenser was replaced by a water separator and 10 mL cyclohexane was added into the condensation reaction mixture, which was then held at 70 °C for 30 min, during which the white solid precipitated from liquid mixture. The resulting solid was then easily separated by a hot filtration higher than 40 °C to yield the recovered catalyst. And the cyclohexane layered from the liquid mixture was easily recovered by a decantation separation. The recovered catalyst and cyclohexane were directly reused in the next run. The amount of residual catalyst in the liquid phase composed of phenols was roughly measured using a DDS-307 Conductivity Meter equipped with a DJS-1C Conductance electrode. And its accurate measurement was achieved using SPECTROBLUE inductively coupled plasma optical emission spectrometry (ICP-OES) with the standard curve method.25,26 0.5 g liquid composed of phenols was dissolved in ultra-pure water and diluted to 50 mL in a volumetric flask. An Optima SPECTROBLUE ICP-OES purged with argon was operated at 1.4 kW RF power. The nebulizer, auxiliary and plasma gas flow rates were 0.8, 0.8 and 12 L min−1, respectively. The sulfur emission signal was registered at 180.731 nm with a 0.1 s integration time. The observed sulfur emission intensity was due to residual hydrosulfate, the calculation of residual rate shown as below:
 | (4) |
3. Results and discussion
3.1 Characterization of alkylammonium hydrosulfates
The detailed 1H NMR spectra shown in Fig. 1–6 (see ESI†) indicate that the prepared catalysts were consistent with the molecular structures of these alkylammonium hydrosulfates. The acid–base titration results shown in Table 1 indicate that these alkylammonium hydrosulfates in water could be ionized to yield the protons to be almost equal to their theoretical ones, illustrating that they have very high quality. The thermal decomposition point of alkylammonium hydrosulfates higher than 250 °C suggests that these catalysts have a good thermal stability.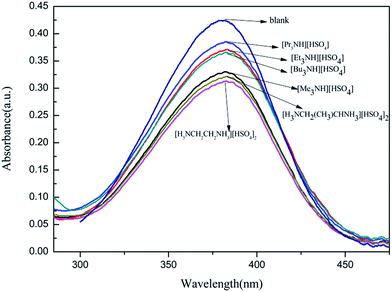 | ||
| Fig. 1 UV-vis absorption spectra in water. Hydrosulfates concentration, 20 mmol L−1; 4-nitroaniline, 5 mg L−1. | ||
| Catalyst | Amount (mmol) | H+ (mmol) |
|---|---|---|
| [Me3NH][HSO4] | 1.01 | 1.00 |
| [Et3NH][HSO4] | 1.00 | 0.98 |
| [Pr3NH][HSO4] | 1.00 | 1.00 |
| [Bu3NH][HSO4] | 1.01 | 0.99 |
| [H3NCH2CH2NH3][HSO4]2 | 0.50 | 1.02 |
| [H3NCH2CH(CH3)NH3][HSO4]2 | 0.51 | 1.04 |
Fig. 1 is the detailed absorption spectra of 4-nitroaniline in the presence of alkylammonium hydrosulfates. In that, a strong absorption band at λmax = 375 nm is found in an UV-vis spectrum of 4-nitroaniline, and the intensity of such band obviously decreases in the presence of alkylammonium hydrosulfates, which should be due to a protonated effect.
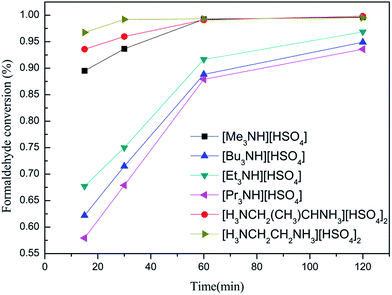
Furthermore, the decaying degree of such band is influenced by the hydrosulfates and follows an increasing sequence of [H3NCH2CH2NH3][HSO4]2 > [H3NCH2(CH3)CHNH3][HSO4]2 > [Me3NH][HSO4] > [Et3NH][HSO4] > [Bu3NH][HSO4] > [Pr3NH][HSO4]. This can be indicative of an acid strength order of the hydrosulfates (inversely proportional to H0 value, see Table 2). The acidic strength of these hydrosulfates seems to be relative to alkyl chains of their quaternary ammoniums and obviously decreases when the alkyl carbon atom of hydrosulfate is more than 3.
| Catalyst | H0 | Formaldehyde conversion at a period of 15 min (%) | Bisphenol selectivity at a period of 15 min (%) | BPF isomer distribution at a period of 15 min (%) | ||
|---|---|---|---|---|---|---|
| p,p′-BPF | o,p′-BPF | o,o′-BPF | ||||
| a Reaction conditions: phenol/formaldehyde, 10 mol mol−1; catalyst (HSO4−)/formaldehyde, 0.05 mol mol−1; temperature = 70 °C. | ||||||
| [Me3NH][HSO4] | 1.48 | 89.5 | 89.8 | 35.3 | 44.4 | 20.3 |
| [Et3NH][HSO4] | 1.73 | 67.7 | 89.1 | 28.1 | 48.7 | 23.2 |
| [Pr3NH][HSO4] | 1.81 | 57.9 | 88.9 | 28.4 | 48.8 | 22.8 |
| [Bu3NH][HSO4] | 1.78 | 62.2 | 89.7 | 28.2 | 48.4 | 23.4 |
| [H3NCH2CH2NH3][HSO4]2 | 1.38 | 96.7 | 90.1 | 36.9 | 43.2 | 19.9 |
| [H3NCH2CH(CH3)NH3][HSO4]2 | 1.47 | 93.6 | 89.9 | 36.5 | 43.4 | 20.1 |
3.2 Catalytic performance of alkylammonium hydrosulfates
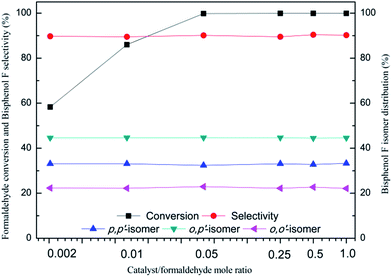
Table 2 lists the data for the hydrosulfates-catalyzed condensation of phenol with formaldehyde. It is seen from Table 2 that these hydrosulfates are very active for this reaction, which can give rise to 57–97% formaldehyde conversion at 15 min with approximately 90% selectivity for BPF. Notably, three hydrosulfates with H0 value of lower than 1.5 exhibits by much higher catalytic activity compared to the other ones with relatively high H0 value. Time-dependence of formaldehyde conversion shown in Fig. 2 also illustrates that the catalytic rate of hydrosulfates is basically consistent with their acidity and the stronger acidity of hydrosulfates is more conducive to accelerate this condensation. This indicates that the stronger acidity of hydrosulfates is more conducive to accelerate this condensation. Table 2 further shows that acidity of the catalyst plays an adjusting effect on the distribution of BPF isomers, when the H0 value of hydrosulfates is reduced from 1.81 to 1.38, the selectivity of p,p′-BPF gradually increases from 28.4 to 36.9%, along with a decrease in the selectivity for other two isomers (o,p′-BPF from 48.8 to 43.2%, and o,o′-BPF from 22.8 to 19.9%), indicating that the stronger acidity of catalyst is favourable of accelerating the formation of p,p′-BPF. The above results indicate that [H3NCH2CH2NH3][HSO4]2 seems to be the optimum catalyst for formation of bisphenol F from condensation of phenol with formaldehyde.In the following experiments, the effect of various parameters including the amount of catalyst, reaction temperature and time, phenol/formaldehyde mole ratio on this condensation was checked in details using the best [H3NCH2CH2NH3][HSO4]2 as a catalyst and the results are shown in Fig. 3–6. It is seen from Fig. 3 that formaldehyde conversion clearly increases with an increase in the amount of catalyst Fig. 4 (catalyst/formaldehyde mole ratio from 0.002 to 0.01), with a further increase in the amount of catalyst (catalyst/formaldehyde ratio from 0.05 to 1.00) yielding a very slow increase in formaldehyde conversion. Moreover, the selectivity for bisphenol F and the distribution for BPF isomers remain almost unchanged over the entire range of catalyst concentration. Moreover, the selectivity for bisphenol F and the distribution for BPF isomers remain almost unchanged over the entire range of the amount of catalyst.
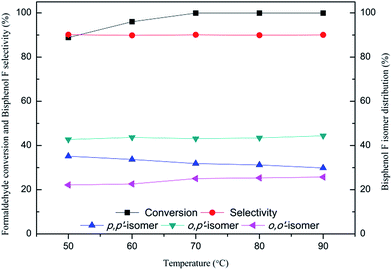
As shown in Fig. 4, increasing the reaction temperature from 50 to 70 °C resulting in a slow increase in formaldehyde conversion from 88.8 to 99.9%. After that, the improved effect of temperature on the conversion is nearly negligible. In the temperature examined, the selectivity for bisphenol F is hardly influenced by temperature. Notably, with the increase of reaction temperature from 50 to 90 °C, the amount of p,p′-BPF is reduced from 35.2 to 29.9%, along with a slight increase in other two isomers p,p′-BPF and o,o′-BPF. This may imply that o,p′-isomer and o,o′-isomer assigned to have a higher thermodynamic stability than p,p′-isomer, which was reported by Jana using aluminium-grafted MCM-41 molecular sieves as catalyst.7
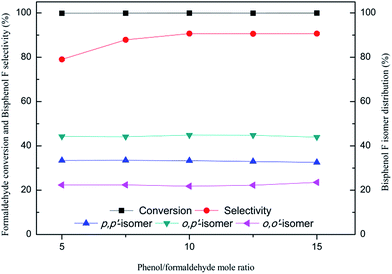
The results shown in Fig. 5 reveal that the mole ratio of phenol to formaldehyde has no significant effect on formaldehyde conversion and the distribution for bisphenol F isomers, but can exert a definite effect on the selectivity for bisphenols F. With the increase of phenol/formaldehyde mole ratio from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the bisphenol F selectivity increase from 79.0% to 90.7%. After that, the selectivity is nearly unchanged with further increasing such ratio.
1, the bisphenol F selectivity increase from 79.0% to 90.7%. After that, the selectivity is nearly unchanged with further increasing such ratio.
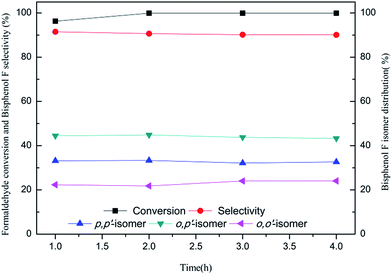
The effect of reaction time on condensation reaction is presented in Fig. 6. With an increase in the reaction time, the formaldehyde conversion and the selectivity of o,o′-BPF increase slightly, while bisphenol F selectivity and the amount of p,p′-BPF and o,p′-BPF decrease slightly. Finally, the catalyst's recovery rate and repeatability were checked using the [H3NCH2CH2NH3][HSO4]2 as a catalyst under the conditions described in the Experimental section and the obtained results are presented in Table 3 and Fig. 7. Table 3 shows that after the catalyst was separated, the measured electro-conductivity of the liquid phase of phenols is close to the electro-conductivity of pure liquid phenol, but drastically less than electro-conductivity of the reaction mixture without separating catalyst, indicating that the residue of catalyst is negligible with such detection method. And the residual rate of catalyst in the liquid of phase phenols determined using the accurate ICP-OES method is in the range of 0.51 to 0.66%, and thereby estimating that the average recovery rate of catalyst is close to 99.5%. Fig. 7 shows that when the catalyst was used repeatedly for 6 times, formaldehyde conversion, bisphenol selectivity and isomer distribution in each recycling run are nearly unchanged compared to those obtained in the first run, this indicates that the catalyst still remained efficient catalytic performance after seven cycle times.
| Recycle time | σ of reaction mixture at 70 °C (μS cm−1) | σ of phenols phase at 25 °C (μS cm−1) | Catalyst residual rate (%) | Sulfur concentration (ppm) |
|---|---|---|---|---|
| a Phenol being crystal state at 25 °C (phenol melts point 40.8 °C). | ||||
| 1 | 48.7 | 0.22 | 0.54 | 0.19 |
| 2 | 47.8 | 0.21 | 0.51 | 0.17 |
| 3 | 48.4 | 0.23 | 0.66 | 0.21 |
| 4 | 47.9 | 0.21 | 0.52 | 0.20 |
| 5 | 47.7 | 0.22 | 0.56 | 0.20 |
| 6 | 47.4 | 0.21 | 0.53 | 0.18 |
| Phenol | 0.21a | — | ||
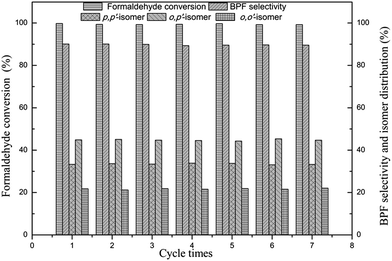 | ||
| Fig. 7 Results of catalyst recycling experiments. Reaction conditions: phenol/formaldehyde ratio, 10 mol mol−1; catalyst/formaldehyde ratio, 0.05 mol mol−1; temperature, 70 °C; reaction time, 2 h. | ||
3.3 The plausible mechanism for formation of bisphenol F
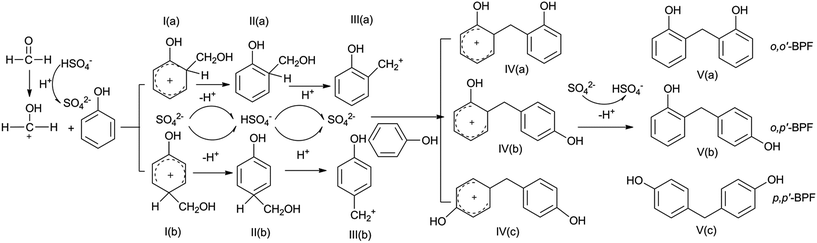
A plausible mechanism of condensation of phenol and formaldehyde to BPF is presented in Scheme 1. A carbocation CH2OH+ is formed by protonation of formaldehyde with H+ released by HSO4− in ethane-1,2-diaminium hydrogen sulfate. An electrophilic substitution reaction occur on phenol with the formation of two intermediates I(a) and I(b) as the first step, then the short-lived intermediates I(b) and I(a) deprotonates fast to form II(a) 2-hydroxybenzylalcohol and II(b) 4-hydroxybenzyl. The protonation of alcohol II(a) and II(b) initially produced form the carbocation III(a) and III(b) respectively. A complexation of the electrophile carbocation III(a) and III(b) with the π electron system of another phenol molecule generates three intermediates IV(a), IV(b) and IV(c): these unstable intermediates then eliminate a proton, resulting in final formation of BPF (V(a) o,o′-BPF, V(b) o,p′-BPF and V(c) p,p′-BPF).4. Conclusions
In this work, a series of water-soluble alkylammonium hydrosulfates have been synthesized conveniently and used for acid-catalyzed condensation of phenol with formaldehyde to bisphenol F. The proposed catalysts are efficient for such reaction, and can achieve formaldehyde conversion of 99.9% and a selectivity of bisphenol F above 89.5% with 33.3% p,p′-BPF, 44.9% o,p′-BPF and 21.8% o,o′-BPF under the optical reaction condition. Furthermore, this type of hydrosulfates can be highly-efficiently recovered and reused for 6 times without any change in catalytic performance. The present catalysis system may be regarded as an environmentally friendly method for the industrial synthesis of bisphenol F at low cost.Acknowledgements
This work is supported by the Graduate Innovation Fund of Hunan province (No. CX2014B170) and National Natural Science Foundation of China (No. 21606082).Notes and references
- L. Pilato, Phenolic Resins: A Century of Progress, Springer, Berlin Heidelberg, 2010 Search PubMed.
- R. O. Morris and M. Wilbraham, US Pat., 4 400 554, 1983.
- R. A. Dubois, H. Ohnishi and A. J. Malzman, US Pat., 5 654 382, 1997.
- M. Okihama and J. Kunitake, JP 08198790, 1996.
- M. Okihama and J. Kunitake, JP 09067287, 1996.
- S. Jana, T. Kugita and S. Namba, Catal. Lett., 2003, 90, 143–147 CrossRef CAS.
- S. K. Jana, T. Kugita and S. Namba, Appl. Catal., A, 2004, 266, 245–250 CrossRef CAS.
- S. K. Jana, T. Okamoto, T. Kugita and S. Namba, Appl. Catal., A, 2005, 288, 80–85 CrossRef CAS.
- A. C. Garade, V. S. Kshirsagar and C. V. Rode, Appl. Catal., A, 2009, 354, 176–182 CrossRef CAS.
- A. C. Garade, V. S. Kshirsagar, R. B. Mane, A. A. Ghalwadkar, U. D. Joshi and C. V. Rode, Appl. Clay Sci., 2010, 48, 164–170 CrossRef CAS.
- R. Liu, X. N. Xia, X. Z. Niu, G. Z. Zhang, Y. B. Lu, R. F. Jiang and S. L. He, Appl. Clay Sci., 2015, 105, 71–77 CrossRef.
- N. Biswal, D. P. Das and K. Parida, J. Chem. Sci., 2014, 126, 455–465 CrossRef CAS.
- Y. Tan, Y. F. Li, Y. F. Wei, Z. M. Wu, J. Q. Yan, L. S. Pan and Y. J. Liu, Catal. Commun., 2015, 67, 21–25 CrossRef CAS.
- A. D. Angelis, P. Ingallina and C. Perego, Ind. Eng. Chem. Res., 2004, 35, 1169–1178 CrossRef.
- Y. H. Zhang, A. L. Zhu, Q. Q. Li, L. J. Li, Y. Zhao and J. J. Wang, RSC Adv., 2014, 4, 22946–22950 RSC.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC.
- A. K. Gupta and R. L. Gardas, RSC Adv., 2015, 5, 46881–46889 RSC.
- Z. N. Siddiqui and K. Khan, ACS Sustainable Chem. Eng., 2014, 2, 1187–1194 CrossRef CAS.
- Z. Zhou and X. Deng, J. Mol. Catal. A: Chem., 2013, 367, 99–102 CrossRef CAS.
- Q. Wang, Z. M. Wu, Y. F. Li, Y. Tan, N. Liu and Y. J. Liu, RSC Adv., 2014, 4, 33466–33473 RSC.
- C. M. Wang, L. P. Guo, H. R. Li, Y. Wang, J. Y. Weng and L. H. Wu, Green Chem., 2006, 8, 603–607 RSC.
- J. Y. Weng, C. M. Wang, H. R. Li and Y. Wang, Green Chem., 2006, 8, 96–99 RSC.
- C. Thomazeau, H. Olivier-Bourbigou, L. Magna, S. Luts and B. Gilbert, J. Am. Chem. Soc., 2003, 125, 5264–5265 CrossRef CAS PubMed.
- F. Reche, M. Garrigós, A. Sánchez and A. Jiménez, J. Chromatogr. A, 2000, 896, 51–59 CrossRef CAS PubMed.
- B. Sinha, V. K. Dakua and M. N. Roy, J. Chem. Eng. Data, 2007, 52, 1768–1772 CrossRef CAS.
- M. Colon, M. Iglesias, M. Hidalgo and J. L. Todolı, J. Anal. At. Spectrom., 2008, 23, 416–418 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07385a |
| This journal is © The Royal Society of Chemistry 2016 |